
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
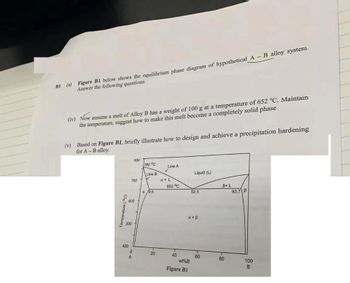
Transcribed Image Text:BI (a) Figure BI below shows the equilibrium phase diagram of hypothetical A - B alloy system.
Answer the following questions.
(iv) Now assume a melt of Alloy B has a weight of 100 g at a temperature of 652 °C. Maintain
the temperature, suggest how to make this melt become a completely solid phase
(v) Based on Figure B1, briefly illustrate how to design and achieve a precipitation hardening
for A-B alloy.
Temperature (°C)
8
700
600
500
400
800
OA
760 °C
Line B
a/9.5
20
Line A
a + L
650 °C
40
Liquid (L)
wt%B
Figure B1
52.5
a + B
60
B+L
80
93.7 B
100
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- In Figure 1 (phase diagram), an alloy composition of 95 wt% Mg and 5 wt% Al is cooled from 700 C to 475 C. An analysis of the resulting ε solid-phase composition reveals that it has a higher Mg content than is anticipated by the phase diagram.a) Explain why this might be the case and how your reasoning results in the higher-than-expected Mg content.b) Provide a sketch showing the likely distribution of Mg content within the volume of ε solid-phase.arrow_forwardReferring to Figure 1, please answer the following [NOTE: SHOW YOUR WORK on Figure 1]:a. provide the specific name for the phase boundary lines denoted by A and B. b. identify the phases present at equilibrium in the phase fields denoted by C, D, and E. c. identify the specific name for the point denoted by F. d. What is the maximum solid solubility of Mg in Al? At what temperature does it occur? e. An Al-Mg alloy (10 wt% Mg, 90 wt% Al) is heated slowly (to insure equilibrium) from a temperature of 200 C: i. At what temperature does the first liquid phase form? ii. What is the composition of the liquid phase at the temperature in part i.? iii. At what temperature does complete melting (no solid phase remaining) occur? iv. What is the composition of the last solid remaining prior to complete melting? f. Using the equilibrium phase diagram of Figure 1, identify the phases present, their compositions, and their relative mass fractions at equilibrium for a 70 wt% Mg – 30 wt% Al…arrow_forwardGiven the equilibrium phase diagram (a) below, briefly describe the mechanisms of precipitation hardening/strengthening of an aluminium alloy making reference to the transitions shown in (b) below to X, X to A, X to D, A to B and A to C. а +0 D B Time (a) (b) - anedua Temperaturearrow_forward
- Consider the binary alloy phase diagram shown schematically below. The left-hand side denotes phase a, and the right-hand side the phase ß. The intermediate region is a coexistence regime with both a and ß phases. The three points 1, 2 and 3 in the coexistence region are at 22.8 wt% A, 28.2 wt% A, and 33.7 wt % A respectively. If the points on the phase boundaries L and R are at 20 wt% A and 39 wt % A, respectively, what is the fraction of a phase at 1, 2 and 3? (This question has only one correct answer) T(°C) A-B binary alloy 1400 Coexistence region 3 1 2 1100 B 20 % 40 % Weight % Aarrow_forwardUsing the isothermal transformation diagram given in Figure 2 for a specific material composition to answer the following (this is NOT an Fe-C steel!). NOTE: Assume that you begin with a single-phase sample of α at 790 C.a. What temperature on the diagram corresponds to the α-to-γ phase transformation temperature that would be found on an equilibrium phase diagram? Explain your answer. b. What temperature would provide the most rapid, isothermal phase transformation of α to γ? What would be the minimum time from onset of the phase transformation to its completion? c. On Figure 2, draw and label a cooling schedule (from 790 C) that will produce a single-phase α material at 100 C. How does the thickness of the piece of material influence your ability to create a single-phase α structure? d. Why does the time to initiate phase transformation become smaller as the temperature is reduced within the temperature range above the “nose” of the TTT diagram? Be specific, what process is…arrow_forwardCorrect and complete solution pleasearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY