
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
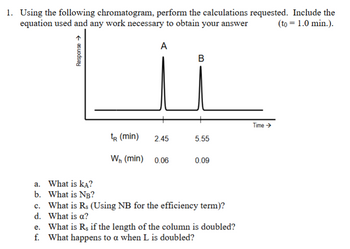
Transcribed Image Text:1. Using the following chromatogram, perform the calculations requested. Include the
equation used and any work necessary to obtain your answer
Response →
A
B
(to = 1.0 min.).
Time →
tR (min)
2.45
5.55
Wn (min)
0.06
0.09
a. What is KA?
b. What is NB?
c. What is Rs (Using NB for the efficiency term)?
d. What is a?
e. What is Rs if the length of the column is doubled?
f. What happens to a when L is doubled?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 10 7 Ruler is in 6 cm unit 5 A B 3 Standards Sample from reaction mixture att = 30 mins Sample from reaction mixture att = 60 mins a. Which type of chromatography based on the polarity of the stationary phase was employed in the experiment? b. Identify the limiting and excess reactants. c. Which product appeared after 30 minutes? d. Which product was produced only after 60 minutes? e. Calculate the retention factor of each reactant and product.arrow_forward-If ethanol (S.G. = 0.790) were used rather than water in measuring the density of the irregular objects, how would that have affected the result? Explain. -When you were measuring the volume of the irregular solid you were told to stir to eliminate bubbles. If the bubbles were not eliminated, how would that affect the density? Explain. -Suppose the density of 65.0 mL of some liquid was found to be 0.748 g/mL. What would be the specific gravity of 45.0 mL of this liquid?arrow_forward28 Report Sheet Separation of the Components of a Mixture D. Determination of Percent Recovery Mass of original sample 10.588 Mass of determined (NH Cl+ NaCl+ SiO2) 1-88 8 Differences in these weights g matter recovered Percent recovery of matter %3D = %00 % g original sample Account for your errors. Combor QUESTIONS 1. Could the separation in this experiment have been done in a different order? For example, if the mixture was first extracted with water and then both the extract and the insoluble residue were heated to dryness, could you determine the amounts of NaCl, NH,Cl, and SiO, originally present? Why or why not? Consult a handbook to answer these questions. 2. How could you separate barium sulfate, BaSO4, from NaCl? 3. How could you separate magnesium chloride, MgCl2, from silver chloride, AgCl? 4. How could you separate tellurium dioxide, TeO2, from SiO,? 5. How could you separate lauric acid from a-naphthol? (See Table 3.1.)arrow_forward
- (8) Identify whether the following are examples of systematic or random error or are mistakes: a. Using an inaccurately calibrated buret. b. An uncertainty of 1 mg in the weight of your sample.∓ c. Overshooting the endpoint on your first sample, then hitting the endpoint on the remaining samples.d. Failing to let your volumetric pipet drain for a few seconds have most of the liquid has been delivered.arrow_forward7. Don believes that fewer than one-third of the students at his college hold part-time jobs while in school. To investigate this, he randomly surveyed 116 students and found that 32 students held part-time jobs. a. ( - Provide a 90% confidence interval for the population proportion of students that hold a part-time job. b. 1 (a). ;Give the correct interpretation of the interval in part c. Extra Credit: Does this interval support Don's claim? Why?arrow_forward(9.29 × 105/8)-20.81 = with correct sig figsarrow_forward
- (lab)1. What is the % yield of recovered caffeine? recovered is 10 mg. (Post lab) 2. What was the % yield of the experiment if there is a possible recovery of 50 mg caffeine? Show calculationsarrow_forwardHow do I complete this problem?arrow_forwardHow do I get the mass of Cu, g produced?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
