
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:of 25 >
© Macmillan Learning
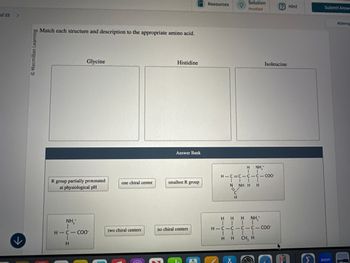
Match each structure and description to the appropriate amino acid.
لا
Glycine
Resources
Solution
Penalized
Hint
Submit Answ
Histidine
Isoleucine
Answer Bank
H
NH₁*
H-C=C C-C-COO
R group partially protonated
at physiological pH
one chiral center
smallest R group
N
NH H H
CII
H
NH₁₂+
H-C-COO
two chiral centers
no chiral centers
E
CH
HHH NH¸*
H-C-C-C-C-COO
| |
HH CH, H
X
Attemp
zoom
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- 22-59 What is the effect of salt bridges on the tertiary structure of proteins?arrow_forwardFor the tripeptide SerValMet a. What amino acid is located at the peptides N-terminal end? b. What amino acid is located at the peptides C-terminal end? c. How many peptide bonds are present? d. How many amide linkages are present?arrow_forwardIsoleucine and threonine are the only two amino acids with two chirality centers. Assign R or S configuration to the methyl-bearing carbon atom of isoleucine.arrow_forward
- 22-91 Which amino acid does not rotate the plane of polarized light?arrow_forwardFor the tripeptide GlyAlaCys a. What amino acid is located at the peptides N-terminal end? b. What amino acid is located at the peptides C-terminal end? c. How many peptide bonds are present? d. How many amide linkages are present?arrow_forward22-42 (a) How many atoms of the peptide bond lie in the same plane? (b) Which atoms are they?arrow_forward
- 22-65 (a) What is the difference in the quaternary structure between fetal hemoglobin and adult hemoglobin? (b) Which can carry more oxygen? (c) What would the oxygen saturation curve of fetal hemoglobin look like compared to that of myoglobin and regular adult hemoglobin?arrow_forwardHemoglobin has pI=6.8. Does hemoglobin have a net negative charge or net positive charge at pH=5.3? At pH=7.3?arrow_forward22-44 How can a protein act as a buffer?arrow_forward
- 22-97 Gelatin is derived from collagen by denaturation. Is a gelatin dessert likely to be a good source of dietary protein?arrow_forward22-48 How many amino acid residues in the A chain of insulin are the same in insulin from humans, cattle (bovine), hogs, and sheep?arrow_forward© Macmillan Learning Match each structure and description to the appropriate amino acid. Glycine Histidine NH₁₂+ NH₁₂+ H HH III H-C-C-C-C-COO- H H CH, H no chiral centers ↓ H-C-COO H one chiral center 0 Resources Solution ? Hint Sub Penalized Isoleucine H NH₁* ↓ H-C=C-C-C-COO IIII ┃┃ -z' N H NH H H smallest R group R group partially protonated at physiological pH two chiral centers Answer Bankarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning