
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
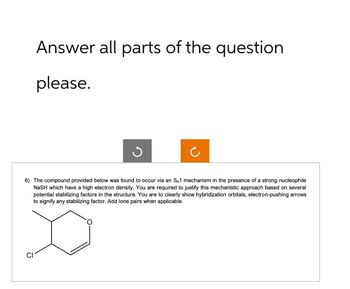
Transcribed Image Text:Answer all parts of the question
please.
ง
6) The compound provided below was found to occur via an SN1 mechanism in the presence of a strong nucleophile
NASH which have a high electron density. You are required to justify this mechanistic approach based on several
potential stabilizing factors in the structure. You are to clearly show hybridization orbitals, electron-pushing arrows
to signify any stabilizing factor. Add lone pairs when applicable.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- See image belowarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided resonance structures, draw the curved electron- pushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bond- breaking and bond-making steps. I I I I :O: farrow_forward2) Provide a detailed mechanism for the addition reaction shown below. State the regiochemistry and explain why it's unusual. Please be sure to include all structures (use line angle notation or perspective diagrams as appropriate to illustrate the stereochemistry of the process), resonance forms, intermkediates, transition states, curved arrows, formal charges, or lone pairs as necessary. Please don't cheat. Br HBr SCH3 SCH + ENarrow_forward
- Complete the mechanism of hydration of alkene below. Please follow curved arrows to predict the resultingstructures in each step.arrow_forwardThis structure has a lower pKa than usual because the conjugate base of the structure has unusual stability. Using the provided resonance structures, draw the curved electron-pushing arrows to show the deprotonation step. Then, draw the curved electron-pushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bond-breaking and bond-making stepsarrow_forwardPlease answer part b and carrow_forward
- 2) Provide a detailed mechanism for the addition reaction shown below. State the regiochemistry and explain why it's unusual. SCH3 HBr Br SCH3 EN Please be sure to include all structures (use line angle notation or perspective diagrams as appropriate to illustrate the stereochemistry of the process), resonance forms, intermediates, transition states, curved arrows, formal charges, or lone pairs as necessary. Please redraw the substratearrow_forwardRationalize the formation of compound B using the arrow-pushing formalism. Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H: EXP CONT Submit Request Answer Br: H UN Br 6:46 PMarrow_forwardLl.116.arrow_forward
- Use the curved-arrow formalism to show how the electrons flow in the resonance form on the left to give the one on the right. Make the ends of your arrows specify the origin and destination of reorganizing electrons. Arrow-pushing Instructions Submit Answer m H Try Another Version 2 item attempts remaining a Use curved arrows to show how the alkene below will react with HCI. Make the ends of your arrows specify the origin and destination of reorganizing electrons. Arrow-pushing Instructions NA ↔XT H Harrow_forwardMechanism 2. Provide the complete mechanism for the reactions below. You must include appropriate arrows, intermediates, and formal charges. The ChemDraw template of this document is available on Carmen. OH H2SO4 (cat) THF OHarrow_forwardGive me detailed mechanism Solution with explanation needed. Don't give Ai generated solution. Avoid handwritten Solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning