
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
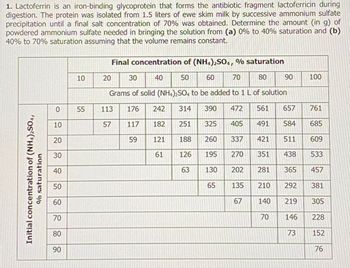
Transcribed Image Text:1. Lactoferrin is an iron-binding glycoprotein that forms the antibiotic fragment lactoferricin during
digestion. The protein was isolated from 1.5 liters of ewe skim milk by successive ammonium sulfate
precipitation until a final salt concentration of 70% was obtained. Determine the amount (in g) of
powdered ammonium sulfate needed in bringing the solution from (a) 0% to 40% saturation and (b)
40% to 70% saturation assuming that the volume remains constant.
Initial concentration of (NH4)2SO4,
% saturation
0
10
20
30
40
50
60
70
80
90
10
55
Final concentration of (NH4)2SO4, % saturation
30
40
50
60
70
80
Grams of solid (NH4)2SO4 to be added to 1 L of solution
176
242 314 390 472 561 657
117
405
491 584
59
421
351 438
20
113
57
182
121
61
251
325
63
188
126 195
130
65
260 337
270
202 281
135
67
210
140
70
90
761
685
511 609
533
365
292
219
146
HUGH LATLE
100
73
457
381
305
228
152
76
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- Gel Running Buffer is made and kept at 14X concentration for storage. We will need 1.5 L of this solution at a concentration of 1X to run our gels. How would we make this solution? I am having trouble understanding the steps to this question, if you could please help me understand it that would be greatly appreciated!arrow_forwardWhat is the minimum length of the column required to completely separate the amino acids? What is the time required for solvent elution (i.e. time for all amino acids to exit the column)?arrow_forwardA spheroidal bacterium with a diameter of 1.0 mm (micrometer, 1 mm = 10-6) contains 25,000 molecules of the protein hexokinase. What is the molar concentration of the protein inside the cell?arrow_forward
- 1.0 0.9 Z 0.8 15. A pharmaceutical company studied the binding of three different compounds, X, Y, and Z, to a particular protein of interest. For each compound, the fractional saturation (v) was determined as a function of concentration. The results are shown in the figure. Which of the three compounds has the strongest affinity for the protein? Explain. 0.7 X 0.6 > 0.5 0.4 0.3 0.2 0.1 0.0 Y 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2.2 2.4 2.6 2.8 3.0 L(μM)arrow_forwardGiven a stock protein solution with a concentration of 6 mg/ml, determine the protein concentration of a solution made by mixing 5 μl of the stock with 5 μl of a buffer.arrow_forward1.0.1 mL of a protein solution of concentration of 11 mg/mL was diluted to a total volume of 4.0 mL with water (i.e. 0.1 mL of the solution was added to 3.9 mL of water). 2 mL of this solution was then mixed with 18 mL of water. What is the concentration of the diluted protein solution? Space to show your workings:arrow_forward
- 2.58. Bacteria have equivalent diameters of 2 × 10-6 m and densities of approximately 1 kg/L. Under optimal conditions, bacteria can di- vide every 30 min. Determine the mass of bacteria that would accumulate in 72 hr under continuing optimal growth conditions. Can this occur? Explain.arrow_forwardcalculate the volume of stock solutions required to make up the buffer solutions that will be used for protein purification. The solutions you need to prepare for purification are: i. Binding Solution A: make up 50 mL 50 mM HEPES buffer (pH 7.5), 300 mM NaCl, 5mM imidazole, 5% (v/v) glycerol ii. Wash Solution B: make up 50 mL 50 mM HEPES buffer (pH 7.5), 300 mM NaCl, 75mM imidazole, 5% (v/v) glycerol iii. Elution Solution C: make up 10 mL 50 mM HEPES buffer (pH 7.5), 300 mM NaCl, 500 mM imidazole, 5% (v/v) glycerol please show your working . Thnk youarrow_forwardA stock solution contains 0.2 mg/mL protein. From this stock solution, 179 uL is diluted with 1.1 mL buffer. What is the concentration of protein in this dilution?arrow_forward
- The α-amylase (α-AM) enzyme secreted by Aspergillus has an isoelectric point of approximately 4.2. If the purification method in problem 1 operates at pH 6.5, would the protein carry a positive charge, negative charge, or net zero charge?arrow_forwardyou are ready to elute your MBP-BAP protein from the column using 1.5mL of TBS + 10mM maltose. Create a buffer from the provided 1x TBS and 500mM maltose stock solution.arrow_forwardWhich of the following is NOT a unit of concentration? All of these options are units of concentration. mM Moles/Liter Molar You gathered your data from Lab , and found the following values: Total Activity of lysozyme in HEW: 14 units Total Activity of lysozyme in carb 1: 21 units Total amount of protein in HEW: 70 mg Total amount of protein in Carb 1: 15 mg Calculate the extent of purification of lysozyme in Carb 1. A 7 fold B 150% C 15 fold D 70%arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON