
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
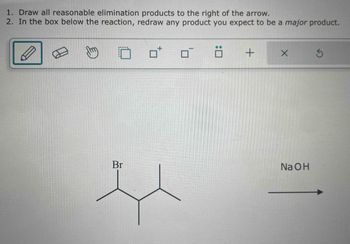
Transcribed Image Text:1. Draw all reasonable elimination products to the right of the arrow.
2. In the box below the reaction, redraw any product you expect to be a major product.
Ö
Br
□
+
X
NaOH
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. A) Show all the unique mono-halogenated products for the reaction below. Circle the major product. Br₂ light B) Show the formal arrow pushing mechanism for the first and second propagation step which leads to the radical intermediate of the major product. Be sure to draw in the hydrogen which is being abstracted. Draw the products.arrow_forwardWhat Is the mnajor product of this reaction? Click on a letter A through D to answer. HoC CHs +BUOK H Et Ph -BUOH H3C H3C H CH3 A. Et Ph Et Ph Et, CH3 B. H3C Ph D. Et Ph C.arrow_forwardWhat major product (from Figure #1) results from the following dehydration reaction (from Reaction #1)? Reaction #1 HO, H2SO4 heat Figure #1 compound A compound B compound C compound D Please click here if image does not display. In predicting the major product of boxed reaction #1, it is strongly recommended that you work out a complete mechanism on a piece of scratch paper. compound C compound A compound D compound Barrow_forward
- For the reaction shown below, circle the major product obtained when the | reaction is allowed to reach equilibrium. Place a box around the product that is formed the fastest. а H2C= CI H-CI CIH2C CIH,C H3C CI CI `CH3 H2C CIH,C H;C CIarrow_forwardHelparrow_forward9. Under normal conditions, 1-cyclohexenol cannot be isolated or stored in a bottle. a. Explain why 1-cyclohexenol cannot be isolated or stored. b. Draw the product that would be isolated and stored instead of 1-cyclohexanol. c. Draw a mechanism to explain your answer above.arrow_forward
- For the reaction shown, draw the product of one mole of reagent adding across the triple bond, and then draw the product of a second mole of reagent. 1 mol HCI 1 mol HCI product A product B Draw product A. Draw product B.arrow_forwarddraw productsarrow_forward6. Circle which of these reactions are viable. aldol product Кон КОн aldol product `H. КОН aldol productarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY