
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
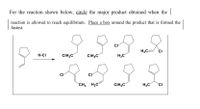
Transcribed Image Text:**Instruction for Reaction Analysis**
For the reaction shown below, *circle* the major product obtained when the reaction is allowed to reach equilibrium. *Place a box* around the product that is formed the fastest.
**Reaction Details:**
1. The starting material is a cyclopentene (five-membered ring with a double bond).
2. The reagent used in the reaction is hydrochloric acid (H-Cl).
3. The products are various chlorinated cyclopentanes, each differing by the position of the chlorine atom and the configuration of additional methyl groups.
**Products:**
- Multiple structures show chlorination at different positions on the cyclopentane ring.
- Some structures feature methyl groups in addition to the chlorine atom.
- The products vary by the position of the chlorine and any additional substituents.
**Task:**
- Identify and *circle* the major product at equilibrium.
- Identify and *box* the product that forms the fastest, which is typically the kinetic product.
Ensure that the analysis considers both thermodynamic stability and kinetic accessibility of the products.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following correctly describes a key step of the reaction shown in the box? 1. ELONA 2 2. HаО, НСІ OEt :o: :o: :o: A) Et H3C p: 1 :o: :o: :o: :o: B) H3C Et H3C :o: :o: :0: + C) H2C H3C :o: H :0: :0: :o: D) H3COEt H3C OEt O A O B OD O=arrow_forward5. Which of the following statements regarding the reactions A and B (shown below) is the best? 0 heat 0 heat B A . Both reaction A and B are feasible because frontier orbitals of reactants can overlap in-phase, allowing the simultaneous formation of two o bonds. . Both reaction A and B are NOT feasible because frontier orbitals of reactants can NOT overlap in-phase. Only reaction A is feasible because frontier orbitals of reactants can overlap in- phase, allowing the simultaneous formation of two o bonds. Only reaction B is feasible because frontier orbitals of reactants can overlap in- phase, allowing the simultaneous formation of two o bonds.arrow_forwardWhich of the following is an intermediate in the reaction below? A) H3t H₂CH H B (Sia)₂ ☆ó B) H₂ (~= UH CH₂CH H H E) H₂E NOH 1. (Sia) 2BH THF 2. NaOH, H₂ de. H D) H3 (1 нarrow_forward
- HO .OH cat. H2SO4 H₂O cat. H2SO4 Complete both mechanisms above. BRIEFLY explain how the different reaction conditions (in the presence of acid) result in different products being formed. How does equilibrium play a role?arrow_forwardSolve reactionarrow_forward2) mny Starting mater 1al and product. Provide condihons for a reaction when givern I. 3) HHarrow_forward
- Draw the step by step reaction mechanism and prerdict products(s) of the following reactionarrow_forwardplease do last 4arrow_forwardMechanism: A reaction mechanism for the following reaction is shown below. H+ CEN CEN: N-H || -C-OH H₂O, H* Step 1 wand woled mot ozsm E Step 3 N-H C-OH C=N-H Step2 C=N-H H-O-H H₂O motno vgiene fesrigid onlt to smotnos -C=N-H a) The overall reaction is an example of b) Step 1 is c) Step 3 is d) Draw in the curved arrows for each step. e) Identify the nucleophiles and electrophiles where appropriate. f) Fill in the reaction energy diagram. H₂O: rate determining step to noipojovo hamwell sit 5(emise erit voittons 10) vanas mi sdgin al rainW di of E^ reaction progress →arrow_forward
- Considering each of the following values and neglecting entropy, tell whether the starting material or product is favored at equilibrium: (a) ?H° = 80 kJ/mol; (b) ?H° = -40 kJ/mol.arrow_forwardWhich of the reactions shown below are correct? & OH oxo H₂O* CO₂H CO₂Et CO₂Et NaOCH₂CH3 HOCH₂CH₂ NaOCH₂CH3 HOCH₂CH3 요 CO₂Et H₂C CH3CO₂Et + CHCO₂Et H₂Carrow_forwardOH CH3-CH2-C-O-CH2-CH3 H OH CH3-CH₂-C-OH HI CH3-CH₂-0-0-CH₂-CH3 ||| Predict the product of the following reaction. + HO-CH2-CH3 H* O-CH2-CH3 CH3-CH2-C-O-CH2-CH3 H || CH3-CH₂-C-CH₂-CH3 IV A) I B) II C) III D) IVarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY