
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
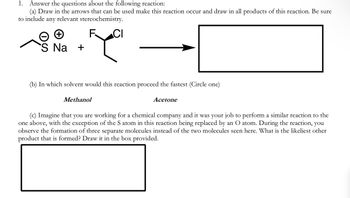
Transcribed Image Text:1.
Answer the questions about the following reaction:
(a) Draw in the arrows that can be used make this reaction occur and draw in all products of this reaction. Be sure
to include any relevant stereochemistry.
+
'S Na +
F
CI
(b) In which solvent would this reaction proceed the fastest (Circle one)
Methanol
Acetone
(c) Imagine that you are working for a chemical company and it was your job to perform a similar reaction to the
one above, with the exception of the S atom in this reaction being replaced by an O atom. During the reaction, you
observe the formation of three separate molecules instead of the two molecules seen here. What is the likeliest other
product that is formed? Draw it in the box provided.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Basic Concept/reaction Worksheets 5.2 Using pen or pencil, draw by hand or e-pen the products of these reactions. (a) H,C- (b) (c) (f) (g) H₂C (h) (i) (e) CH₂CHC=C(CH₂), CH, OH (CH₂),COOH, OO₂(cat) (CH₂),COH. HO™ OH HO OH H₂SO heat C₂Hs H CH₂CCH₂CH=CHCH₂CCH, OH O₂N 1. LIAIH, diethyl ether 2. H₂O OH 1. B₂H. diglyme 2. H₂O₂, HO™ + NO₂ H₂CrO H₂SO4, H₂O, acetone NO₂ O₂N 1. LiAlH4, diethyl ether 2. H₂O 0 0 ii + CH₂COCCH3 - CCI CH₂OH H₂SO4 pyridinearrow_forwardanswer question c only. and please further explain about this topic. Thank you in advance!arrow_forwardGive answer all questions with explanation pleasearrow_forward
- If Zaitsev's rule does not apply and the base is bulky then how will the product look?arrow_forwardiii) 2-Bromo-2-cyclopropylpropane will undergo an SN1 reaction called solvolysis in methanol to give several products, two of which are shown below. Use curly arrows to show how the formation of these two products occurs mechanistically.arrow_forwardPlease don't provide handwriting solutionarrow_forward
- QUESTION 2a between one equivalent of HBr with the compound shown below? What would be the major 1,2 and 1,4 products formed by the reaction Indicate which is the kinetic and which is the thermodynamic product and the reasoning for your choice (1-2 sentences maximum). Draw an energy diagram to help explain your choice. intermediates. Also, how would it is possible to favor formation of the 1,4 over 1,2 product and the Show the complete arrow pushing mechanism for the formation of each product and all 1,2 over 1,4 product. HBr (1 eq)arrow_forwardDo not give handwriting solution.arrow_forward43C\ ......cion 2 (a) What reaction will occur if a catalytic amount of the base sodium ethoxide is added to acetone? Propose a structure of the product from this reaction. HK CH₂ NaOEt (0.05 eq) (b) What product would be expected if acetone were added to an excess of a stronger base (e.g. LDA)?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY