
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
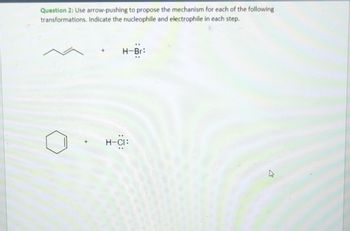
Transcribed Image Text:Question 2: Use arrow-pushing to propose the mechanism for each of the following
transformations. Indicate the nucleophile and electrophile in each step.
H-Br:
H-CI:
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part A Draw the product formed when the structure shown below undergoes substitution with OH™. Interactive 3D display mode Br H CH3 Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. Show the appropriate stereochemistry by choosing the dashed or wedged buttons and then clicking a bond on the canvas.arrow_forwardivatives: Nucleophilic Acyl Substitution Reactions - EOC [References] From the table of available reagents select the one(s) you would use to convert butanoic acid to each of the following products: (Use the minimum number of steps; from one to six are required. List reagents by letter in the order that they are used; example: fa. NOTE: Assume that a normal aqueous (or mild acid) workup follows each reaction; you do not need to add reagents for the workup.) Reagents Available a. benzene/AlCl3 d. H2O, H2SO4 g. LiAlH4 j. PBг3 b. (CH3)2CuLi e. H₂, Pd/C h. NaCN C. CH3NH2 f. K* -OC(CH3)3 i. NH3 k. Dess-Martin periodinane I. SOCI₂ 1-butanol butanenitrilearrow_forwardSection A: Apply your knowledge 1. Decide whether the following nucleophiles would react with a ketone, aldehyde, or both and draw the corresponding product(s). If a reaction seems unfavorable explain why/how a product can still be obtained. H₂O Or MeOH Or O HO OH Or H2N—Ph Or H3C CH3 Or 1.1 HO HN—CH3 Orarrow_forward
- Nitesharrow_forwardIn the following acid-base reactions, 1. draw Lewis structures of the reactants and the products. 2. determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases). 3. use the curved-arrow formalism to show the movement of electron pairs in these reactions, as well as the imaginary movement in the resonance hybrids of the products. 4. indicate which reactions are best termed Brønsted-Lowry acid-base reactions. (c) -BH₁₂ BH3 + CH3-O-CH3 → CH3-O-CH3 (d) 유 0- CH3-C H + -OH CH₁₂-C-H OHarrow_forwardWhat alkene is needed to synthesize the following 1,2-diol using the given reagents? Be sure to answer all parts. CH3CH₂CH2 [1] OsO4 followed by NaHSO3 in H₂O: [2] CH3CO3H followed by "OH in H₂O: H OH OH CH₂CH₂CH3 draw structure... draw structure ...arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY