
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
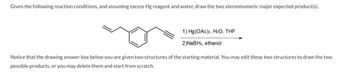
Transcribed Image Text:Given the following reaction conditions, and assuming excess Hg reagent and water, draw the two stereoisomeric major expected product(s).
1)
2) NaBH, ethanol
Hg(OAc)2, H:O, THF
Notice that the drawing answer box below you are given two structures of the starting material. You may edit these two structures to draw the two
possible products, or you may delete them and start from scratch.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- draw the major productsarrow_forwardPart A Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. CH₂ Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. Show the appropriate stereochemistry by choosing the dashed or wedged buttons and then clicking a bond on the canvas. CONT? NN 1. CH,COH 2. H, H₂O DC ⓇH: 2D A" EXP OH CH₂ OH H H с N O S 25 Br - P Farrow_forwardIII. Predict the Product. For each of the following reactions, please identify the type of reaction favored (either SN 1, S N 2, E1, E2) and draw the major product(s). If there is no reaction, please indicate as "NR." Note: be sure to clearly show product stereochemistry! d) f) Br Br Br NaOEt HOEt H₂O "warm" H₂O Δarrow_forward
- A common alkene starting material is shown below. Predict the major product or missing reagent to complete each reaction. Use a dash or wedge bond to indicate stereochemistry, where applicable. Ignore inorganic byproducts.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. :0: H H Ⓒ:0 Select to Add Arrows HCI, CH3CH2OH I 1 II -H I Iarrow_forwardanswer does not include dashes or wedgesarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw any missing organic intermediates or products for this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts and counterions. H 0:0- HsC H Select to Add Arrows H HaC Select to Add Arrows CH₂OH, H CH3OH, H* CH3OH, H* HaC H Select to Add Arrows 1 CH3OH, H+ HaC H H Select to Add Arrows Iarrow_forward#18arrow_forwardiii) 2-Bromo-2-cyclopropylpropane will undergo an SN1 reaction called solvolysis in methanol to give several products, two of which are shown below. Use curly arrows to show how the formation of these two products occurs mechanistically.arrow_forward
- help please #1arrow_forwardPlease don't provide handwriting solutionarrow_forwardMoving to another question will save the reporse Question 22 Using the pool of reagents provided below (table) complete the statement regarding the reaction below Fill in the blanks The following transformation can be accomplished by using the corresponding to the reagents from the reagents pool below set of reagents Write the letter in UPPERCASE AICHAL ZICul A) CH₂l2, Z(Cu) B) PhMgBr 2 H3O* C) H₂SO4, H₂O D) Na, NH3 E) Cl₂, CC, 1 mol F) HgSO4, H₂SO4, H₂O G) HBr, 1 mol H) B2, CC, 1 mol I) HCl, 1 mol J) Sia BH; 2) H₂O2, NaOH KI PhMaBrexcess 2) H.O K) PhMgBr excess, 2) H₂0* L) HCl, excess (M) Ch, CCI, excess N) HBr, peroxide O) Lindlar's catalyst P) Pd, H₂, ethanol Q) 03; 2) H₂O R) KF, Acetone S) KF, H₂O T) BHS. THF 2) H₂O2, NaOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY