
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
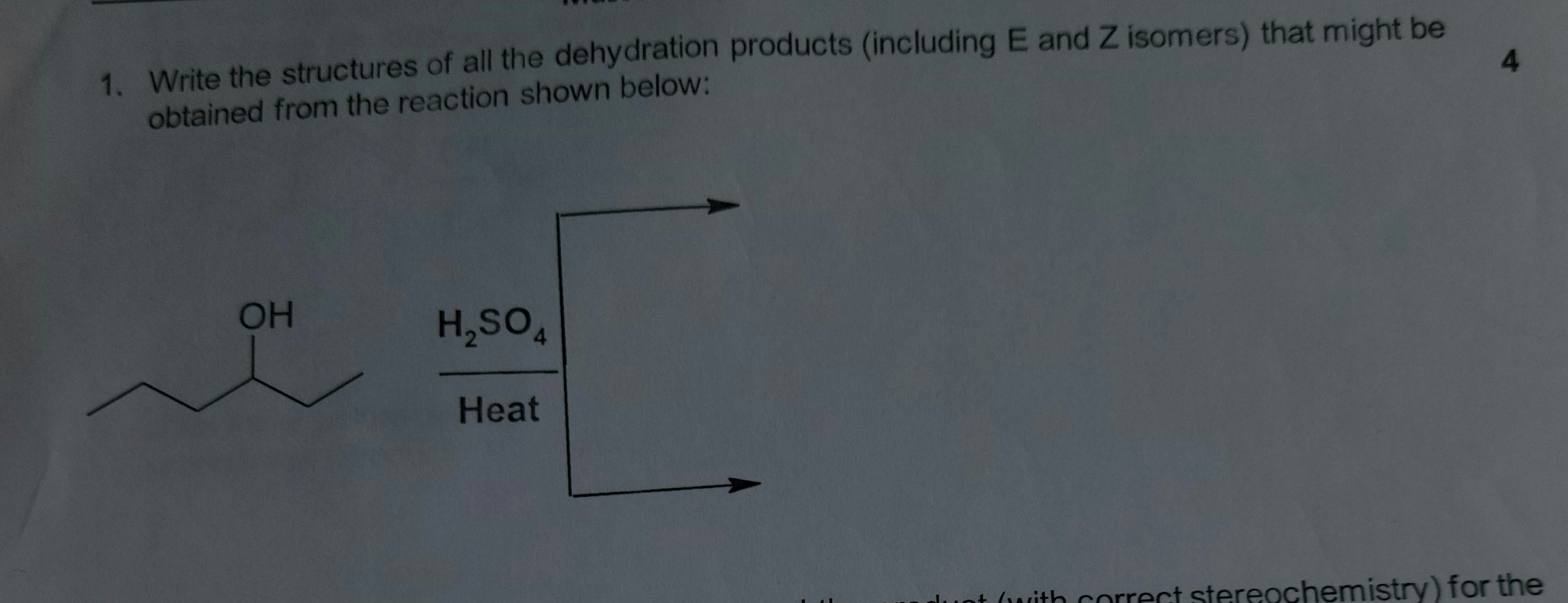
Transcribed Image Text:1. Write the structures of all the dehydration products (including E and Z isomers) that might be
obtained from the reaction shown below:
OH
H,SO,
Heat
luot (with correct stereochemistry) for the
4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the products of this organic reaction: ? CH3-C-O-CH2-O-CH3 nghi KOH Specifically, in the drawing area below draw the structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Click anywhere to draw the first atom of your structure. C C Xarrow_forwardWhy can’t both B and D produce the three desired products? How are they different when becoming carbocations?arrow_forward3. Give the IUPAC name (indicating stereochemistry when necessary) for the following four alkenes: HO Give detailed Solutionarrow_forward
- Assign Z or E to the following alkenesarrow_forwardAn alkene having the molecular formula C6H₁2 is treated sequentially with ozone (03) and zinc/acetic acid to give the product/s shown. O Draw a structural formula for the alkene. H3C 1 • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + ▾ CH3 will n [F ChemDoodleⓇ 16arrow_forwardFor each reaction below suggest structures for alkenes that give the indicated reaction products. There may be more than one answer in some casesarrow_forward
- (a) 2-methyl-2-pentanol Draw the structure of the alkene that was used to prepare the alcohol in highest yield. AAVIL ? ChemDoodleⓇ Which process does this employ? 1. Hg(OAc)2, H₂O; 2. NaBH4 01. BH3; 2. H₂O2, NaOH OsO4, H₂O2 On n [ ]#arrow_forward4. Purpose the synthesis for the following transformations (e) COOH CI Br NO2arrow_forwardDraw a structural formula for the major organic product of the reaction shown below.arrow_forward
- ОН CH3CH2CH2CH2ČHCH3 Can this alcohol be synthesized selectively by hydroboration-oxidation of an alkene? • If yes, draw the structure of the alkene. • If no, draw the structure given above. • You do not have to explicitly draw H atoms. opy aste ChemDoodlearrow_forwardUse the References to access important values if needed for this question. Draw the structure of the organic product of the following dehydration reaction. If more than one product is possible, draw the structure of the major product. tosH heat CH • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. P. opy aste [片 CH4 Previous Ne ChemDoodle" Saarrow_forwardComplete each of the reactions below with the predominant product or products. You must indicate stereochemistry with wedges and dashes. If a racemic mixture is created, you must draw both structures and write "racemic" if appropriate. Br Br JI... Br je Br H3C- NaOH Na NaCl N3 Na CN KOtBuarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY