
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
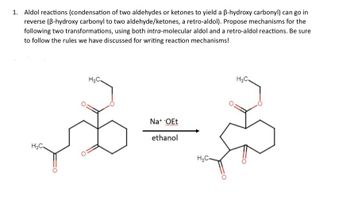
Transcribed Image Text:1. Aldol reactions (condensation of two aldehydes or ketones to yield a ẞ-hydroxy carbonyl) can go in
reverse (B-hydroxy carbonyl to two aldehyde/ketones, a retro-aldol). Propose mechanisms for the
following two transformations, using both intra-molecular aldol and a retro-aldol reactions. Be sure
to follow the rules we have discussed for writing reaction mechanisms!
H₂C.
H&C.
Na+ -OEt
ethanol
H₂C-
H₂C.
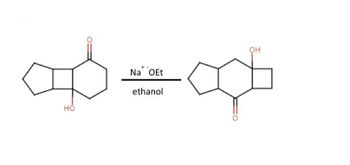
Transcribed Image Text:HO
Na OEt
ethanol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- I H H3C NOC XT :0: MET H₂C base H3C The aldol reaction is a carbonyl condensation reaction between two carbonyl partners and involves a combination of nucleophilic addition and a-substitution steps. One partner is converted into an enolate ion nucleophile and adds to the electrophilic carbonyl group of the second partner. In the classic aldol reaction, the carbonyl partners are aldehydes or ketones, although aldehydes are more reactive. The product is a ß-hydroxy carbonyl compound. H OH q H3C H₂C H Under reaction conditions slightly more vigorous than those employed for the aldol reaction, the ß-hydroxyl group is eliminated in an E1cB dehydration to give an a,ß-unsaturated carbonyl compound. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions heat H H + H₂Oarrow_forward2 H3C H3C H C→XT OH H3C The aldol reaction is a carbonyl condensation reaction between two carbonyl partners and involves a combination of nucleophilic addition and a-substitution steps. One partner is converted into an enolate ion nucleophile and adds to the electrophilic carbonyl group of the second partner. In the classic aldol reaction, the carbonyl partners are aldehydes or ketones, although aldehydes are more reactive. The product is a ß-hydroxy carbonyl compound. base :0: OH H H Under reaction conditions slightly more vigorous than those employed for the aldol reaction, the ß-hydroxyl group is eliminated in an E1cB dehydration to give an a,ß-unsaturated carbonyl compound. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instruct ns H3C heat OH H3C :0: H + H₂O Harrow_forward2 Moin H3C H H₂C C⇒x= base :0: OH Hori H3C H. The aldol reaction is a carbonyl condensation reaction between two carbonyl partners and involves a combination of nucleophilic addition and a-substitution steps. One partner is converted into an enolate ion nucleophile and adds to the electrophilic carbonyl group of the second partner. In the classic aldol reaction, the carbonyl partners are aldehydes or ketones, although aldehydes are more reactive. The product is a B-hydroxy carbonyl compound. Under reaction conditions slightly more vigorous than those employed for the aldol reaction, the ß-hydroxyl group is eliminated in an E1cB dehydration to give an a,ß-unsaturated carbonyl compound. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions H₂C Ἡ :0: heat H Home H3C + H₂Oarrow_forward
- Show all the processes in the mechanism of the reaction.arrow_forward10. Give the mechanism for the aldol reaction of the given enolate and acetaldehyde, show the aldol product. NB: Show all arrows. Li OTBS H م کی داماد Me OTBS Me Enolate o=/ 26arrow_forward4. Provide a mechanism for the following reaction. H₂SO4, H₂O HgSO4 enarrow_forward
- A ketone is produced from an alkyne by addition of water. Draw the enol intermediate and the mechanism of following reaction.arrow_forwardPlease complete the following self-Claisen and self-aldol reactions by drawing structures for the missing reactants or products as needed. Thank you!arrow_forwardComplete the reaction scheme below. Show all reagents and intermediates. No reaction is a possible answer. 1) CH3CH2M9B CH3OH H+ (ехcess) но 2) H30*, H2O B A OCH3arrow_forward
- base 2 H3C H H3C OH heat H H3C + H₂O H The aldol reaction is a carbonyl condensation reaction between two carbonyl partners and involves a combination of nucleophilic addition and a-substitution steps. One partner is converted into an enolate ion nucleophile and adds to the electrophilic carbonyl group of the second partner. In the classic aldol reaction, the carbonyl partners are aldehydes or ketones, although aldehydes are more reactive. The product is a ẞ-hydroxy carbonyl compound. Under reaction conditions slightly more vigorous than those employed for the aldol reaction, the ẞ-hydroxyl group is eliminated in an E1CB dehydration to give an a,ẞ-unsaturated carbonyl compound. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions X :OH H₂O: а کی H₂C H H₂C H Harrow_forward7. Propose a mechanism for the following transformation. H* `NH2arrow_forward4. Draw the aldehydes needed to prepare the compound below by an aldol reaction. OH CHO From: ogynu abmu and Voarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning