
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
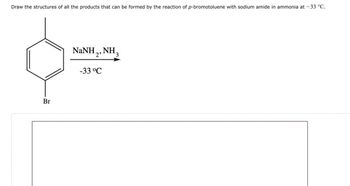
Transcribed Image Text:Draw the structures of all the products that can be formed by the reaction of p-bromotoluene with sodium amide in ammonia at -33 °C.
Br
NINH, NH,
'2'
-33°C
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- What is the major organic product obtained from the following reaction? -- تمرة AICI 3 ب ب میں مجھ IV I Oarrow_forwardH3C OH OH H+ CH3 Esters can be synthesized by an acid-catalyzed nucleophilic acyl substitution between an alcohol and a carboxylic acid; this process is called the Fischer esterification reaction. Because the alcohol oxygen is a poor nucleophile, the carbonyl carbon is made a better electrophile by protonation of the carbonyl oxygen. The steps of the synthesis are all reversible. The reaction is generally driven to completion by using an excess of the liquid alcohol as a solvent, or by distilling off the product as it forms. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions CIX 10-4 H₂O CH3 H₂O CH3arrow_forwardKindly give me complete answers thanks..arrow_forward
- Draw the major organic product obtained by reaction of benzoyl chloride with dimethylamine, (CH3)2NH.arrow_forwardH₂C ཏཱཏི 1 ནི OH 1. Br2, PBг3 2. H₂O H3C OH Br The a-bromination of carbonyl compounds by Br2 in acetic acid is limited to aldehydes and ketones because acids, esters, and amides don't enolize to a sufficient extent. Carboxylic acids, however, can be a-brominated by first converting the carboxylic acid to an acid bromide by treatment with PBr3. Following enolization of the acid bromide, Br2 reacts in an a-substitution reaction. Hydrolysis of the acid bromide completes the reaction. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions :0: H3C Br Br + :::OH2 Br H₂O H3C Br заarrow_forwardWhat is the major organic product obtained from the following reaction under low temperature conditions? A, B, C, or D?arrow_forward
- Draw the skeletal structures of all the final organic products formed when phenol (C6H-OH) is treated with the set of reagents given below: OH HNO₂ H₂SO4 1) Sn, HC1 2) dilute aq NaOH ?arrow_forwardDraw the structural formula of the enol formed in each alkyne hydration reaction; then draw the structural formula of the carbonyl compound with which each enol is in equilibriumarrow_forwardProvide the reagents necessary to convert 3-methyl-2-butanol to 2-bromo-3-methylbutane. CHOOSE FROM THE FOLLOWING REAGRENTS BELOW conc. HBr NaBr, H2SO4 PBr3 HBr, peroxide Br2arrow_forward
- Claisen condensation between diethyl phthalate and ethyl acetate followed by saponifi- cation, acidification, and decarboxylation forms a diketone, C,H,O,. COOEt 1. EtO Na+ HCI, H,O B NaOH, H,O + CH3COOEt C,H,O2 2. НС, Н,О heat heat COOEt Diethyl phthalate Ethyl acetate Propose structural formulas for compounds A and B and the diketone.arrow_forwardWhat is the major organic product obtained from the following reaction? H HC A) 1 B) 2 HO H₂SO4 C) 3 OH 2 سل، ز. مل D) 4 OHarrow_forward1. NaOEt CO₂Et 2. H3O+ 3. heat Br H3C CO₂H CO2 H3C CO₂Et The malonic ester synthesis is a carbonyl alkylation reaction. It is used to prepare carboxylic acids from primary alkyl halides, lengthening the carbon chain by two atoms. Thus, the product can be visualized as being a "substituted acetic acid." The reaction consists of three steps: generation of the enolate anion followed by SN2 reaction with a primary alkyl halide, ester hydrolysis under acid conditions, and decarboxylation. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions 22 CX EtO₂C EtO₂C HOCH2CH3 HOCH2CH3 EtO₂C EtO₂Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
