
EBK A SMALL SCALE APPROACH TO ORGANIC L
4th Edition
ISBN: 9781305446021
Author: Lampman
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Question
Please provide a mechanism for the reaction with P-bromophenacyl ester. The unknown is 3-pentanone.
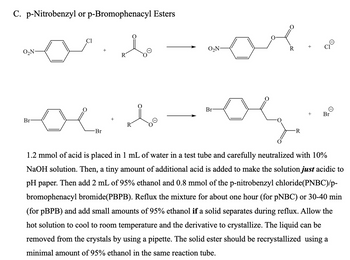
Transcribed Image Text:C. p-Nitrobenzyl or p-Bromophenacyl Esters
Cl
O₂N-
R
Br
ос
R
Br
O₂N-
R
Br
-R
Θ
Br
1.2 mmol of acid is placed in 1 mL of water in a test tube and carefully neutralized with 10%
NaOH solution. Then, a tiny amount of additional acid is added to make the solution just acidic to
pH paper. Then add 2 mL of 95% ethanol and 0.8 mmol of the p-nitrobenzyl chloride(PNBC)/p-
bromophenacyl bromide(PBPB). Reflux the mixture for about one hour (for pNBC) or 30-40 min
(for pBPB) and add small amounts of 95% ethanol if a solid separates during reflux. Allow the
hot solution to cool to room temperature and the derivative to crystallize. The liquid can be
removed from the crystals by using a pipette. The solid ester should be recrystallized using a
minimal amount of 95% ethanol in the same reaction tube.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 42 segundos) ga X KY Inglés What is the purpose that in the reactions of carboxylic acids with alcohol they have an excess of acid if there is already an acid in solution (carboxylic acid)? 1.so that the solution has an excess of hydroxyls 2.to increase pH due to carboxylic acid 3.so that the solution Onigle Tra Págin: El Trac traducc por God video er Fecha de Propietar Tambierarrow_forwardmark mechnism of catalysisarrow_forwardE OH F HO HO OH OH 1. H2SO4, toluene, heat excess dimethyl ketone 2. CrO3/HCI/Py/DCM 3. H3O+, H₂O 2R-methylhexanal 1. Ammonia 2. hydrogen cyanide 3. acidic hydrolysis under refluxarrow_forward
- 2,4-Pentanedione is a considerably stronger acid than is acetone (Chapter 19). Write a structural formula for the conjugate base of each acid and account for the greater stability of the conjugate base from 2,4-pentanedione.arrow_forwarda. Compound X is benzene, Y is acetic anhydride acid. Complete the following scheme and determine Z! b. Determine which reagents except acetic acid anhydrides can replace Y!arrow_forwardO NaBH4 Synthesis #2: Select a different set of two substrates and one reagent that could be combined to prepare benzphetamine. tymen Ph Ph Ph Ph NH Ph H O NaBH3CN, H+ 20= ano [H*], NaBH-CN Nucleophilearrow_forward
- 1) CO2 (s) g) Ph(CH:)MgBr 2) 11 (aq) H'(aq)/A h) 6-Bromohexanoic Acid + NACN- 1) NaOEt/ELOH i) PhCOHC-CH2 + CH:(CO Et) 2) H (aq), A,-CO. Butanoic Acid 1 equivalent Bry/P H.NEt k) Methyl Benzoate NaOMe/MEOHI I) 2 (Ph) CHCOOME -arrow_forwardRank the derivatives of phenol in order of increasing acidity (Please explain) arrow_forwarddont provide handwriting solution ...arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
