
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
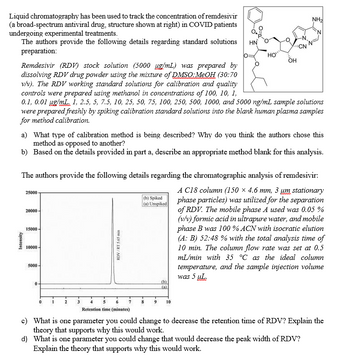
Transcribed Image Text:Liquid chromatography has been used to track the concentration of remdesivir
(a broad-spectrum antiviral drug, structure shown at right) in COVID patients
undergoing experimental treatments.
Intensity
The authors provide the following details regarding standard solutions
preparation:
HN
CN
HO
OH
NH2
Remdesivir (RDV) stock solution (5000 µg/mL) was prepared by
dissolving RDV drug powder using the mixture of DMSO: MeOH (30:70
v/v). The RDV working standard solutions for calibration and quality
controls were prepared using methanol in concentrations of 100, 10, 1,
0.1, 0.01 µg/mL. 1, 2.5, 5, 7.5, 10, 25, 50, 75, 100, 250, 500, 1000, and 5000 ng/mL sample solutions
were prepared freshly by spiking calibration standard solutions into the blank human plasma samples
for method calibration.
a) What type of calibration method is being described? Why do you think the authors chose this
method as opposed to another?
b) Based on the details provided in part a, describe an appropriate method blank for this analysis.
25000
20000-
(b) Spiked
(a) Unspiked
The authors provide the following details regarding the chromatographic analysis of remdesivir:
A C18 column (150 x 4.6 mm, 3 um stationary
phase particles) was utilized for the separation
of RDV. The mobile phase A used was 0.05%
(v/v) formic acid in ultrapure water, and mobile
phase B was 100% ACN with isocratic elution
(A: B) 52:48 % with the total analysis time of
10 min. The column flow rate was set at 0.5
mL/min with 35 °C as the ideal column
temperature, and the sample injection volume
was 5 μL.
15000-
10000
5000-
0
RDV/RT 5.65 min
(b)
www
(a)
T
T
T
2
3
4
5
6
7
8
9
10
Retention time (minutes)
c) What is one parameter you could change to decrease the retention time of RDV? Explain the
theory that supports why this would work.
d) What is one parameter you could change that would decrease the peak width of RDV?
Explain the theory that supports why this would work.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- ow do the properties of a nonhomogeneous (heterogeneous) mixture differ from those of a solution? Give two examples of nonhomogeneous mixtures.arrow_forwardA solution is made by dissolving 34.0 g of NaCl in 100 g of H2O at 0C. Based on the data in Table 8-1, should this solution be characterized as a. saturated or unsaturated b. dilute or concentratedarrow_forwardA solution is made by dissolving 0.455 g of PbBr2 in 100 g of H2O at 50C. Based on the data in Table 8-1, should this solution be characterized as a. saturated or unsaturated b. dilute or concentratedarrow_forward
- 6-54 An industrial wastewater contains 3.60 ppb cadmium Cd2+. How many mg of Cd2+ could be recovered from a ton (1016 kg) of this wastewater?arrow_forwardCalicheamicin gamma-1, C55H74IN3O21S4, is one of the mostpotent antibiotics known: one molecule kills one bacterialcell. Describe how you would (carefully!) prepare 25.00 mLof an aqueous calicheamicin gamma-1 solution that couldkill 1.0 x108 bacteria, starting from a 5.00 x 10-9M stocksolution of the antibiotic.arrow_forwardythos.content.blackboardcdn.com/5af05a7aa8cfe/3542526?X-Blackboard-Expiration=1614816000000&X-E 100% Suppose you dissolve 4.50 g of NAOH in 500. mL of methanol, which has a density of 0.791 g/mL. The solution has a total volume of 505. mL. Find the: moles solute (a) Molarity = Liters of solution moles solute (b) Mole fraction= moles solute plus moles solvent moles solute (c) Molality= kg solvent (d) Mass % solute= mass solute X 100 mass of solute plus mass of solvent (e) ppm = Parts per million= mass solute X 1,000,000 mass of solute plus mass of solvent 18 HB #2arrow_forward
- You have a 0.50M stock of glucose, a 150mM stock of asparagine, and a 0.05M stock of NaH2PO4, and magnesium chloride powder (solid form), MgCl2 (formula weight: 95.21g/mole). How would you prepare (be specific) 25mL of a solution that contains 0.05M glucose, 15mM asparagine, 2.5mM NaH2PO4 and 50mM MgCl2?arrow_forward(12) At a given temperature the vapor pressures of benzene and toluene are 183 mm Hg and 59.2 mm Hg, respectively. Calculate the total vapor pressure over a solution of benzene and toluene with Xbenzene = 0.580. A) 106 mm Hg B) 121 mm Hg C) 131 mm Hg D) 242 mm Hg E) 262 mm Hgarrow_forward> EL session.masteringchemistry.com MasteringChemistry ROSTER-2019-SPRI-PULLM-CHEM-101 blackboard wsu Google Search MasteringChemistry: HW 8 KHW 8 Applications for Dilution Calculations K8 of8 Dilution as a procedure in analysis We sometimes purposely dilute solutions if the analyte concentrations wavelengths in the visible (and sometimes uitraviolet) range. Based on the dissolved solute's properties, we can quantify that solute's concentration are too high to permit an accurate measurement. For example, a spectrophotometer can measure the amount of light that passes through a sample solution for If the solute's concentration is too high, a relatively small amount of incident light having the wavelength that corresponds to the solute's absorption behavior will less light is absorbed, and the detector in the spectrophotometer can generate more reliable data. reach the dete ctor, which results in an inaccurate measurement (consider the result if you blocked the light with an opaque…arrow_forward
- Indicate the appropriate protocol below to prepare the required solution. a) Solution 5 Preparation: Prepare: 10 mL of 23% mannitol From: solid mannitol Procedure: b) Solution 2 Preparation: Prepare: 0.5 liters of 0.01 mg/mL ascorbic acid From: solid ascorbic acid , MW = 176.1 g/mol Procedure: C) Solution 3 Preparation: Prepare: 1 liter of 150 mM Tris From: 1 Molar Tris stock solution Procedure:arrow_forwardmass/ - H. Hm X180) d x 100 (Q16) What is the mass percent of NaCIO in 0.97M bleach sample? (Molar mass of NaCIO=74.5 g/mol, density of bleach solution= 1.08 g/mL) A) 0.73 % B) 7.2% C) 0.070 % D) 6.7% E) 0.97% the molar mass of a volatile liquid experiment, if the pressure reading from thearrow_forwardDensity of solution:Trial 1: 1.2 g/mLTrial 2: 1.2 g/mLTrial 3: 1.2 g/mL Average density = 1.2 g/mL What is the relative average deviaion, %?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning




Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning