
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
Assign all the protons
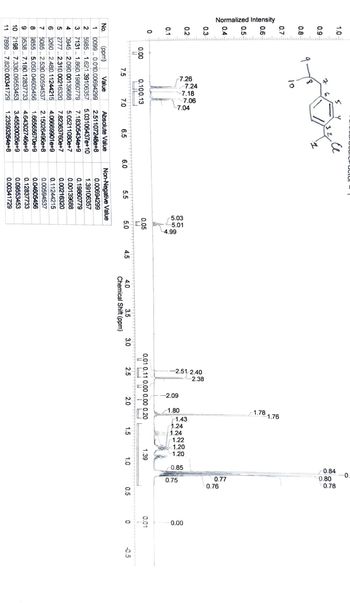
Transcribed Image Text:0.1
0.2
0.6
Normalized Intensity
0.3
0.4
0.5
-7.26
0.9
1.0
0.8
10
0.7
7.24
-7.18
7.06
-7.04
8
6
3 2
1
Се
-5.03
-5.01
4.99
-2.51-2.40
-2.38
0.84
-0.
0.77
0.80
0.76
0.78
1.78
1.76
1.43
1.24
1.24
1.22
1.20
1.20
0.85
0
0.00
0.100.13
0.05
0.01 0.11 0.00 0.00 0.20
1.39
0.01
ㅂ
الا
ㅂ
7.5
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
3.0
2.5
2.0
1.5
1.0
0.5
0
-0.5
Chemical Shift (ppm)
No.
(ppm) Value
Absolute Value
Non-Negative Value
1
0099.. 0.010.00694299
2.51107248e+8
0.00694299
2
5985.. 1.621.39106357
5.03106437e+10
1.39106357
3
7131.. 1.860.19860779
7.18305434e+9
0.19860779
4
0945. 2.090.00139688 5.05211080e+7
0.00139688
5
2777.. 2.310.00216320
7.82363760e+7
0.00216320
6
3260.. 2.480.11244215
4.06669901e+9
0.11244215
7
5085.. 2.530.00594537 2.15026496e+8
0.00594537
8
9855.. 5.050.04605456
1.66565670e+9
0.04605456
9
9538.. 7.180.12837733
4.64302746e+9
0.12837733
10 2198.. 7.330.09553453
3.45520026e+9
0.09553453
11 7899.. 7.820.00341729
1.23593264e+8
0.00341729
-2.09
1.80
0.75
0.00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Please help to.identify all peaksarrow_forwardTHE SLOPE OF THE LINE IN THE FOLLOWING GRAPH IS: In K -27 -35.0 O.974 -35.2 -35.4 -35.6 -35.8 -36.0 -10749 -36.2 I In K = -AH"/RT+AS" IR In K* = -10,749/T-27 R² = 0.974 7.4 7.6 7.8 8.0 (1/7)/10-4K-1 8.2 O NOT ENOUGH INFORMATION 8.4 8.6arrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forward
- Apply the Q test to the following data sets to determine whether the outlying result should be retained or rejected at the 95% confidence level. a. 85.10, 84.62, 84.70b. 85.10, 84.62, 84.65, 84.70arrow_forwardMolar concentration of the dye stock solution: 2.52e-5 mol/L Operating Wavelength: 630 nm Solutions(Dilutions) Absorbance (A)(a.u.) 1 0.073 2 0.118 3 0.258 4 0.477 Using linear regression determine the absorbance/concentration relationship for the dye. [dye] =_____ x Aarrow_forwardTHE Y-INTERCEPT FOR THE FOLLOWING GRAPH IS: -35.0 In K# -10749 -27 -35.2 -35.4 -35.6 -35.8 -36.0 -36.2 7.4 In K = -AH/RT+AS*/R In K* = -10,749/T-27 R² = 0.974 7.6 7.8 974 NOT ENOUGH INFORMATION 8.2 8.0 (1/7)/10-4K-1 8.4 8.6arrow_forward
- Please create a caption for this table. Solution NaCl Conc. (%) Osmolality (mOsm) % transmittance Absorbance % hemolysis % crenation C distilled 0 0 0.001029 4.987584625 100 0.03354 1 0.177179111 54.61 0.001551 4.809388202 96.42720001 0.05837 2 0.297126222 91.58 0.01012 3.994819487 80.09527231 0.08444 3 0.442542222 136.4 3.849 1.414652089 28.3634704 0.134 4 0.590164444 181.9 64.8 0.188424994 3.777880643 0.2125 5 0.74752 230.4 95.64 0.019360433 0.388172513 0.3368 6 0.89644 276.3 99.56 0.001915112 0.038397585 0.5336 7 1.095648889 337.7 99.98 8.68676E-05 0.001741676 0.9834 8 1.336711111 412 100 0 0 2.1 9 1.755568889 541.1 100 0 0 7.9 10 2.674395556 824.3 100 0 0 57.83 11 4.490211111 1384 100 0 0 99.72arrow_forward20,18,2,4,15,15,10 Using these data, construct a 80% confidence interval for the average net change in a student's score after completing the course. Assume the population is approximately normal. Construct the 80% confidence interval. Round your answer to one decimal place.arrow_forwardLearning AA prod03-cnow-owl.cengagenow.com Login Learning Learning × Online tea... y dr. marlow... ember L... with We 3. AS surroundi... M 2BrF3 (9) Br2 (g) + 3F2 (9) 4. AG° = AH°... M 5. AG: Pre... 1req 6. AG: Enthal... Using standard thermodynamic data at 298 K, calculate the free energy change when 2.14 moles of BrF3 (9) react at standard conditions. Substance AG (kJ/mol) 7. AG fro... 1req Question Question Question 8. Calculat... 1req AG° = 9. Calculat... 1req BrF3 (9) -229.4 Br2(g) 3.1 F2(9) 0.0 kjarrow_forward
- Can you help me label this irarrow_forwardALEKS - Jacqueline Hoppenrey x G convert mg to g - Google Searc x A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQiHqRdYV 6Ux63Syp.JXz0Coxvwqgg4JkWI72X79QvOLp9_7U27sYQhkaocvdwecGvsUzo65uy3F6spORRg1XSqgh81is O STOICHIOMETRY Using molarity to find solute mass and solution volume Jacqueline A chemist adds 55.0 mL of a 4.75M silver perchlorate (AGCIO,) solution to a reaction flask. Calculate the mass in grams of silver perchlorate the chemist has added to the flask. Round your answer to 3 significant digits. Explanation Check Privacy Accessibil 2021 McGraw-Hill Education All Rights Reserved Terms of Usearrow_forwardWhere am I going wrong? The collision cross section for neon is 0.24 nm2.Calculate the mean free path (in nanometers), between collisions for neon atoms at a pressure of 1.00 atm and a temperature of 31°C. λ = _______ nm.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

