
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
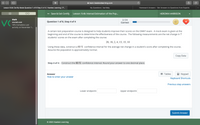
20,18,2,4,15,15,10
Using these data, construct a 80% confidence interval for the average net change in a student's score after completing the course. Assume the population is approximately normal.
Construct the 80% confidence interval. Round your answer to one decimal place.
Transcribed Image Text:A learn.hawkeslearning.com
Lesson 9.4b Certify Mode Question 1 of 8 Step 4 of 4 | Hawkes Learning | P...
My Questions | bartleby
Homework Answers - Get Answers to Questions from Experts
+
+ Save & Exit Certify
Lesson: 9.4b Interval Estimation of the Pop...
HERONN HARRISON
Math
2/24
VC
covcel.com
Question 1 of 8, Step 4 of 4
Correct
4
14% Complete Last
activity on November 1..
A certain test preparation course is designed to help students improve their scores on the GMAT exam. A mock exam is given at the
beginning and end of the course to determine the effectiveness of the course. The following measurements are the net change in 7
students' scores on the exam after completing the course:
20, 18, 2, 4, 15, 15, 10
Using these data, construct a 80 % confidence interval for the average net change in a student's score after completing the course.
Assume the population is approximately normal.
Copy Data
Step 4 of 4: Construct the 80 % confidence interval. Round your answer to one decimal place.
Answer
E Tables
Кeypad
How to enter your answer
Keyboard Shortcuts
Previous step answers
Lower endpoint:
Upper endpoint:
Submit Answer
© 2020 Hawkes Learning
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I don't know this question?arrow_forwardHP TrueVision HD Pavil cal App AN Careers 4 Texas Workfor Mộc Homework X W College Physic agleonline.hccs.edu/courses/188795/assignments/3601808 mabie diter rep 4 dl 10.17am Homework Ch16 - Attempt 1 Problem 16.35 11 of 15 > Constants | Periodic Table Complete the third row. Express your answer using two significant figures. Complete the following table by calculating the missing entries and indicating whether the solution is acidic or basic. Acidic or [H+] [OH-] pH pOH basic? ΑΣφ 7.4x10-3 [OH ] 3.3x10-10 M 8.25 Submit Request Answer 5.73 hp ODOLE ADVANCED fa f9 ho 4+ fn 10 f12. insertarrow_forwardHow do I solve for the percent value? The sample data calculated the answers but I have no idea how they obtained the percent error values. Calculate sample data #1 with detailed steps.arrow_forward
- Consider the following operations on the number 8.83 x 10-². Without using a calculator, decide which would give a significantly smaller value than 8.83 x 10-2, which would give a significantly larger value, or which would give essentially the same value. 8.83 x 10-2 +3.23 x 105 (same 8.83 x 10-2-3.23 x 105 smaller 8.83 x 10 2 x 3.23 x 105 larger v 8.83 x 10-2/3.23 x 105 smallerarrow_forwardQ7. You obtain the set of results 192, 216, 202, 195, and 204. Using Grubbs Test at 95% confidence level, should the value 216 be retained or rejected?arrow_forwardPerform the calculations and determine the absolute and percent relative uncertainty. Express each answer with the correct number of significant figures. To avoid rounding errors, do not round your answers until the very end of your calculations. 7.8 (+0.3) – 2.03 (±0.02) = absolute uncertainty: percent relative uncertainty: 8.24 (+0.04) × 0.012 (±0.001) = absolute uncertainty: percent relative uncertainty: %arrow_forward
- Find the weighted average of these values. Value Weight 5.00 75.0% 6.00 15.0% 7.00 10.0% weighted average:arrow_forwardIn the following sub-questions, use your book and notes to generate an argument for each model of light. Remember, on the first day of class we discussed that argumentation requires: ● a claim (which I've given), • evidence (which you should look up in the form of data or scientific principles) and reasoning that connects the evidence to the claim (which you should generate from your understanding so far).arrow_forwardCan you help me answer this questions 4 and 5, this is from the previous question I just split my question, thanksarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY