
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
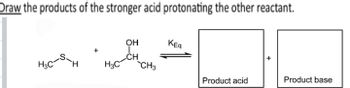
Transcribed Image Text:Draw the products of the stronger acid protonating the other reactant.
OH
KEq
CH
H3C
H3C
`CH3
Product acid
Product base
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Several acids and their respective equilibrium constants are: Which is the strongest acid? Which is the weakest acid? Which acid has the weakest conjugate base? Which acid has the strongest conjugate base?arrow_forwardAnswer true or false to the following statements about the mechanism of acid-base reactions. (a) The acid and base must encounter each other by a collision in order for the proton to transfer. (b) All collisions between acids and bases result in proton transfer. (c) During an acid-base reaction the lone pair on the base fills the A-H antibonding sigma orbital.arrow_forwardIn each equilibrium, label the stronger acid, the stronger base, the weaker acid, and the weaker base. Also estimate the position of each equilibrium. (a) CH3CH2O + CH3CCH CH3CH2OH + CH3CC (b) CH3CH2O + HCl CH3CH2OH + Cl (c) CH3COOH + CH3CH2O CH3COO + CH3CH2OHarrow_forward
- Predict the position of equilibrium for this acid-base reaction.arrow_forwardComplete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reaction. (a) (b) (c) (d)arrow_forwardWrite an equation to illustrate the equilibrium that is present when propanoic acid is dissolved in water. What structure predominates when OH is added to raise the pH to 12? What structure predominates as acid is added to lower the pH to 2?arrow_forward
- For the following reaction, the products are favored at equilibrium. Classify each of the reactants and products based on their strength as Bronsted-Lowry acids or bases.C5H11N + C6H5COOHC5H11NH+ + C6H5COO- C6H5COO- C5H11N C5H11NH+ C6H5COOH Stronger Bronsted-Lowry acid Weaker Bronsted-Lowry acid Stronger Bronsted-Lowry base Weaker Bronsted-Lowry basearrow_forwardExplain why an acid-base reaction will only proceed if the products formed are weaker conjugate acid and base compared to the starting acid and base.arrow_forwardFor the following acid-base reaction, predict which side of the equilibrium is favored. OH favor right side favor left side neither + H₂O + + H₂Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning