
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 6.SE, Problem 45AP
Interpretation Introduction
Interpretation:
Given methoxide ion reacts with bromoethane in a single step as shown.
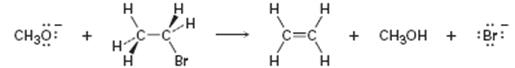
The bonds broken and formed are to be identified and curved arrows are to be drawn to show the flow of the electrons.
Concept introduction:
Curved arrows start from a nucleophilic source (neutral or negatively charged) and end in an electrophilic sink (neutral or positively charged). During the flow of the electrons if one bond is broken other has to be formed so as to maintain the octet rule.
To identify:
The bonds broken and formed in the reaction given and to draw curved arrows to show the flow of the electrons.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
85. Propane, C3H8, is a hydrocarbon that is commonly used as a fuel.
(a) Write a balanced equation for the complete combustion of propane gas.
(b) Calculate the volume of air at 25 °C and 1.00 atmosphere that is needed to completely combust 25.0 grams of propane. Assume that air is 21.0 percent O2 by volume. (Hint: We will see how to do this calculation in a later chapter on gases—for now use the information that 1.00 L of air at 25 °C and 1.00 atm contains 0.275 g of O2 per liter.)
(c) The heat of combustion of propane is −2,219.2 kJ/mol. Calculate the heat of formation, ΔH∘fΔHf° of propane given that ΔH∘fΔHf° of H2O(l) = −285.8 kJ/mol and ΔH∘fΔHf° of CO2(g) = −393.5 kJ/mol.
(d) Assuming that all of the heat released in burning 25.0 grams of propane is transferred to 4.00 kilograms of water, calculate the increase in temperature of the water.
Write down a balanced chemical equation for the complete combustion of methanol CH3OH(I) to CO2(g) H2O(I)
Draw the skeletal structure of the product(s) for the Lewis acid-base reaction.
Chapter 6 Solutions
Organic Chemistry
Ch. 6.1 - Prob. 1PCh. 6.3 - Prob. 2PCh. 6.3 - Using curved fishhook arrows, propose a mechanism...Ch. 6.4 - Prob. 4PCh. 6.4 - An electrostatic potential map of boron...Ch. 6.5 - What product would you expect from reaction of...Ch. 6.5 - Reaction of HBr with 2-methylpropene yields...Ch. 6.6 - Prob. 8PCh. 6.6 - Predict the products of the following polar...Ch. 6.7 - Which reaction is more energetically favored, one...
Ch. 6.7 - Prob. 11PCh. 6.9 - Which reaction is faster, one with ∆G‡ = +45...Ch. 6.10 - Prob. 13PCh. 6.SE - Prob. 14VCCh. 6.SE - Prob. 15VCCh. 6.SE - Prob. 16VCCh. 6.SE - Look at the following energy diagram: (a) Is...Ch. 6.SE - Look at the following energy diagram for an...Ch. 6.SE - What is the difference between a transition state...Ch. 6.SE - Prob. 20EDRMCh. 6.SE - Prob. 21EDRMCh. 6.SE - Draw an energy diagram for a two-step exergonic...Ch. 6.SE - Draw an energy diagram for a reaction with keq =...Ch. 6.SE - The addition of water to ethylene to yield ethanol...Ch. 6.SE - When isopropylidenecyclohexane is treated with...Ch. 6.SE - Prob. 26EDRMCh. 6.SE - Draw the electron-pushing mechanism for each...Ch. 6.SE - Draw the complete mechanism for each polar...Ch. 6.SE - Prob. 29EDRMCh. 6.SE - Identify the functional groups in the following...Ch. 6.SE - Identify the following reactions as additions,...Ch. 6.SE - Identify the likely electrophilic and nucleophilic...Ch. 6.SE - For each reaction below identify the electrophile...Ch. 6.SE - Prob. 34APCh. 6.SE - Follow the flow of electrons indicated by the...Ch. 6.SE - Prob. 36APCh. 6.SE - Prob. 37APCh. 6.SE - Despite the limitations of radical chlorination of...Ch. 6.SE - Prob. 39APCh. 6.SE - Answer question 6-39 taking all stereoisomers into...Ch. 6.SE - Prob. 41APCh. 6.SE - Prob. 42APCh. 6.SE - Prob. 43APCh. 6.SE - The reaction of hydroxide ion with chloromethane...Ch. 6.SE - Prob. 45APCh. 6.SE - Ammonia reacts with acetyl chloride (CH3COCl) to...Ch. 6.SE - The naturally occurring molecule α-terpineol is...Ch. 6.SE - Prob. 48APCh. 6.SE - Prob. 49APCh. 6.SE - Draw the structures of the two carbocation...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In a time-resolved picosecond spectroscopy experiment, Sheps, Crowther, Carrier, and Crim (Journal of Physical Chemistry A, Vol. 110, 2006; pp. 30873092) generated chlorine atoms in the presence of pentane. The pentane was dissolved in dichloromethane, CH2C12. The chlorine atoms are free radicals and are very reactive. After a nanosecond the chlorine atoms have reacted with pentane molecules, removing a hydrogen atom to form HCl and leaving behind a pentane radical with a single unpaired electron. The equation is Cl (dcm) + C5H12(dcm) HCl(dcm) + C5H11 (dcm) where (dcm) indicates that a substance is dissolved in dichloromethane. Measurements of the concentration of chlorine atoms were made as a function of time at three different concentrations of pentane in the dichloromethane. These results are shown in the table. (a) Determine the order of the reaction with respect to chlorine. (b) Determine whether the reaction rate depends on the concentration of pentane in dichloromethane. If so, determine the order of the reaction with respect to pentane. (c) Explain why the concentration of pentane in dichloromethane does not affect the data analysis that you performed in part (a). (d) Write the rate law for the reaction and calculate the rate of reaction for a concentration of chlorine atoms equal to 1.0 M and a pentane concentration of 0.23 M. (e) Sheps, Crowther, Carrier, and Crim found that the rate of formation of HCl matched the rate of disappearance of Cl. From this they concluded that there were no intermediates and side reactions were not important. Explain the basis for this conclusion.arrow_forwardYou have been tasked to assess a new laboratory for safety issues. The lab has a large amount of benzene, hydrochloric acid, phenol, sodium hydroxide and toluene. Describe the (5) potential hazards that can be associated with each chemical above, as well as the methods to control their potential hazards in the lab.arrow_forward6. (Chapter 15-Q37) Indole is an aromatic heterocyclic that has a benzene ring fused to a pyrrole ring. Answer the following questions. Indole 6(a) What is the hybridization of N in this molecule? = 6(b) How many pi electrons N contributes to the ring? = 6() Which orbitals contribute to form a sigma bond between N and H in this molecule? = 6(c) What is the electronic relationship of Indole to naphthalene? Give the answer by comparing number of rings and number of pi electrons in both compounds, write x rings, y pi electrons=|arrow_forward
- Describe the intermediate that is thought to form in theaddition of a hydrogen halide to an alkene, using cyclohexeneas the alkene in your description.arrow_forwardWith the catalyst AlCl3 present, which reactant is needed to convert benzene to ethylbenzene?arrow_forwardWrite a balanced chemical equation based on the following description: propanol, C₃H₇OH(l) burns in airarrow_forward
- Propane, C3H8, is a hydrocarbon that is commonly used as a fuel.(a) Write a balanced equation for the complete combustion of propane gas.(b) Calculate the volume of air at 25 °C and 1.00 atmosphere that is needed to completely combust 25.0 grams of propane. Assume that air is 21.0 percent O2 by volume. (Hint:use the information that 1.00 L of air at 25 °C and 1.00 atm contains 0.275 g of O2 per liter.)(c) The heat of combustion of propane is −2,219.2 kJ/mol. Calculate the heat of formation, ΔH°f of propane given that ΔH°f of H2O(l) = −285.8 kJ/mol and ΔH°f of CO2(g) = −393.5 kJ/mol. (d) Assuming that all of the heat released in burning 25.0 grams of propane is transferred to 4.00 kilograms of water, calculate the increase in temperature of the water.arrow_forwardConsider the compound 2,4-dimethyl-3-isopropylpentane. How many unique radical structures can be derived from this hydrocarbon when it undergoes free radical substitution? Write the number of free radicals formed.arrow_forward) The reaction of hydroxide ion with chloromethane to yield methanol and chloride ion is an example of a general reaction type called a nucleophilic substitution reaction: HO¯ + CH;CI = CH;OH + CI¯ The value of AHº for the reaction is -75 kJ/mol, and the value of ASº is +54 J/ (K- mol). What is the value of AG° (in kJ/mol) at 298 K? Is the reaction exothermic or endothermic? Is it exergonic or endergonic?arrow_forward
- 2) Complete the reaction: NO. LOH FeCLarrow_forward2) Complete the reaction: NO. LOH FECLTarrow_forwardComplete the reaction scheme below by drawing the missing structure. If there is no structure possible that would produce the outcome shown, indicate that fact in the answer file that you submit. N-H 1) NaOH, H₂O 2) Br NaOH, H₂O heat "NH₂ oNa Naarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning