
a)
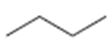
Interpretation:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of n-butane are to be drawn.
Concept introduction:
In radical chlorination reactions, if all the hydrogens present in the alkane are of same kind then it is possible to get a single monochloro product. If the alkane has hydrogens of different kinds then hydrogens belonging to all kinds will be substituted by chlorine resulting in a mixture of monochloro
To draw:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of n-butane.
b)
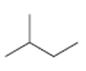
Interpretation:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of 2-methylbutane are to be drawn.
Concept introduction:
In radical chlorination reactions, if all the hydrogens present in the alkane are of same kind then it is possible to get a single monochloro product. If the alkane has hydrogens of different kinds then hydrogens belonging to all kinds will be substituted by chlorine resulting in a mixture of monochloro alkanes as product.
To draw:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of 2-methylbutane.
c)
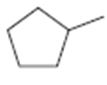
Interpretation:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of methylcyclopentane are to be drawn.
Concept introduction:
In radical chlorination reactions, if all the hydrogens present in the alkane are of same kind then it is possible to get a single monochloro product. If the alkane has hydrogens of different kinds then hydrogens belonging to all kinds will be substituted by chlorine resulting in a mixture of monochloro alkanes as product.
To draw:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of methylcyclopentane.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry
- Fill the blank space. Compounds containing a phenol group may work as ANTIOXIDANTS to prevent free radical damage. This is accomplished when a free radical (or UV light) encounters a phenol group, turning the phenol group into a radical. However, contrary to typical radical behavior, the structure of the phenol radical can neutralize (or quench) the unpaired electron. Specifically, the phenol structure neutralizes (or quenches) the unpaired radical electron by doing the following: taking the electron and ---------. The correct name (or abbreviation) of an example compound containing a phenol group with antioxidant properties is: ---------.arrow_forwardThe compound below is treated with N-bromosuccinimide (NBS) in the presence of light. H Draw both resonance structures for the radical produced by reaction of the compound with a bromine atom. Assume reaction occurs only at the weakest C-H bond. • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. Separate resonance structures using the symbol from the drop-down menu. Include all valence radical electrons in your answer. 0 3 ChemDoodleⓇ Q4 On [] Z Carrow_forwardWhy does Hammett Equation only apply to meta and para substituted rings and not others? Explainarrow_forward
- In theory, there are only three inequivalent hydrogens in this molecule that could be substituted by Br in a free radical bromination – circle them. Put an asterisk to mark the one most likely to be substituted first.arrow_forwarda) Free radical bromination is more selective than free radical chlorination. Draw a reaction coordinate diagram for the specific step in the radical chain mechanism that illustrates the source of this selectivity, and explain your reasoning. b) Explain why the bond dissociate energy (BDE) of tert-butane is 95 kcal/mol while the BDE for propane is 99 kcal/mol.arrow_forwardWhat are the organic products of these reactions? Are they electrophiles or nucleophiles? What is their decreasing order of rate?arrow_forward
- Draw all the different monochlorinated products one would obtain by the radical chlorination of methylcyclopentanearrow_forwardof The triphenylmethyl radical is highly delocalized by resonance. In the resonance hybrid, how many different positions have radical character?arrow_forwardDraw all the different monochlorinated products one would obtain by the radical chlorination of 2-methylbutane.arrow_forward
- For each of the following, write the major product(s) and then draw out each step in the mechanism using curved arrows. Show ALL lone pair electrons and formal charges. Redraw ALL molecules as to show explicitly ALL bonds being broken or formed. Identify the molecular orbital (HOMO) of the nucleophile and the molecular orbital (LUMO) of electrophile involved in the nucleophilic attack. MO diagrams are not necessary..arrow_forwarda. How many monochlorination products can be obtained from the radical chlorination of methylcyclohexane? Disregard stereoisomers.b. Which product would be obtained in greatest yield? Explain.c. How many monochlorination products would be obtained if all stereoisomers are included?arrow_forwardA Moving to another question will save this response. Question 5 Which one of the following compounds does not undergo a Friedel-Crafts reaction? (remember: limitations to this reaction) O toluene O benzene O chlorobenzene O nitrobenzene O t-butylbenzene A Moving to another question will save this response.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
