
Concept explainers
(a)
Interpretation:
All lone pairs to the atom at the point of attachment for every substituent in Table 23-1 are to be added. Also, out of the two columns, the column (ortho/para- or meta directing substituents) in which these substituents can be found is to be given.
Concept introduction:
The substituent on the benzene ring prior to substitution dictates the regiochemistry of the reaction. Some substituents are designated as ortho/para directors because they lead to product mixtures consisting primarily of the ortho- and para-disubstituted products. Other substituents are designated as meta directors because they favor the formation of the meta-disubstituted product. The results from nitration reactions are summarized in Table 23-1:
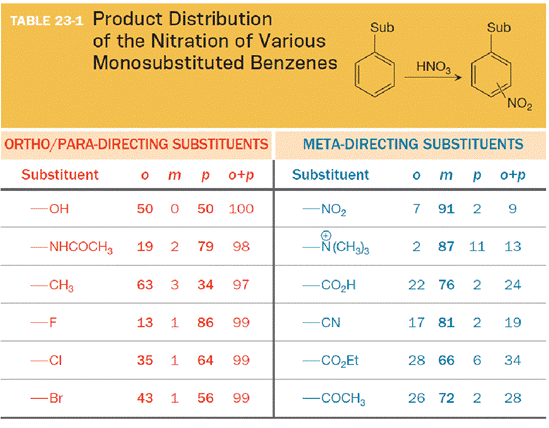
(b)
Interpretation:
The substituent in the column attached by an atom that has no lone pairs of electrons is to be shown. Also any electronegative atoms at or near the point of attachment are to be shown.
Concept introduction:
The substituent on the benzene ring prior to substitution dictates the regiochemistry of the reaction. Some substituents are designated as ortho/para directors because they lead to product mixtures consisting primarily of the ortho- and para-disubstituted products. Other substituents are designated as meta directors because they favor the formation of the meta-disubstituted product. The results from nitration reactions are summarized in Table 23-1:
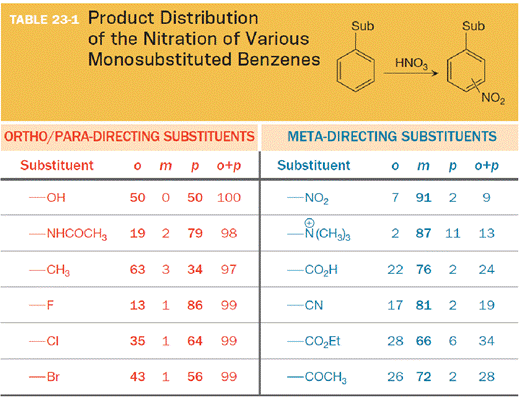
(c)
Interpretation:
In the other column, electronegative atoms at or near the point of attachment are to be found.
Concept introduction:
The substituent on the benzene ring prior to substitution dictates the regiochemistry of the reaction. Some substituents are designated as ortho/para directors because they lead to product mixtures consisting primarily of the ortho- and para-disubstituted products. Other substituents are designated as meta directors because they favor the formation of the meta-disubstituted product. The results from nitration reactions are summarized in Table 23-1:
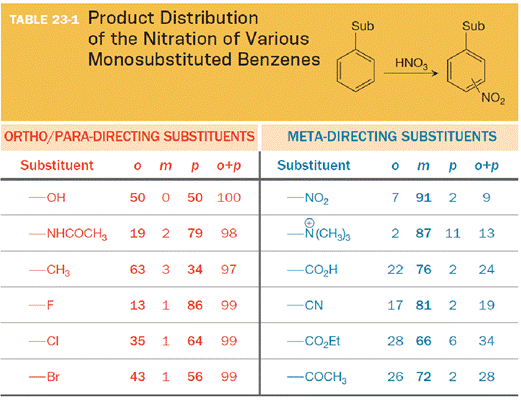
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- (a) What functional group is undergoing a transformation in the reaction? (b) What functional group is it being transformed into (in the final product)?arrow_forwardNoting the curved arrows, draw all the product(s), organic and inorganic, of the following reaction. H3C-C H HH -H + :Ö-H H • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate structures with + signs from the drop-down menu.arrow_forward(a) Re-draw the following molecule with the CH2CH3 group on the ladder 1. H. H2 CF H3C H2 CH2 H;C Is the molecule in part (a) the same molecule or the enantiomer of the following. Circle your answer Explain your answer CH3 CH2 H,C SAME MOLECULE CH3 ENANTIOMER H2arrow_forward
- Click on all structures that are enantiomers of the first (leftmost). If no structure qualifies, submit your answer without selecting any structure. CO-H CO,H H CO2H "נco ווא CH2OH HOH,C H `NH2 CH2OH H2N HOH2C H H2N NH2 Submit Answer Retry Entire Group 2 more group attempts remainingarrow_forwardAnswer the following problem.arrow_forwardCompare the inductive effect and resonance effect in each of the following substituents and determine their combined effect (Table 2). -NH₂ -OH -Me I -COOH -NO₂ Inductive Effect Weak withdrawing Table 2 Resonance effect Strong donating Combined Effect Electron donatingarrow_forward
- (CH;CH,)NH | =Q=Q₂ CI Hexane (COCI)₂2 DMF OH Hexanearrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Н ют 11*** Но Br Explanation Molecule 1 Н c Br ОН Molecule 4 S НО "Н Br - Onone of the above Check - Q Search Molecule 2 ***** Br Н H- ОН Molecule 5 ""Br OH Molecule 3 Br Н H "" OH О Molecule 6 OH *** Br X 3 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility ?arrow_forwardStarting from the wedge-and-dash structure below (sighting down the indicated bond), rotate the back carbon to provide the structure in the conformation that will be capable of an E2 elimination. R/S stereochemistry is graded. H CI K H H3C |||| Drawing < |||| Q Atoms, Bonds and Rings Charges Tap a node to see suggestions. H H H H H Undo X Reset Remove Done Rotatearrow_forward
- Click on all structures that are enantiomers of the first (leftmost). If no structure qualifies, submit your answer without selecting any structure. CO,H H CO2H CO2H CH2OH HOH,C H H וו `NH2 CH2OH H2N HOH2C H H2N NH2 Submit Answer Retry Entire Group 2 more group attempts remainingarrow_forwardA sulfonium ion (R3S+) is a stereogenic center if three different alkyl groups are bonded to sulfur because sulfur is surrounded by four different groups, including its lone pair. In assigning an R or S) is a stereogenic center if three different alkyl groups are bonded to sulfur because sulfur is surrounded by four different groups, including its lone pair. In assigning an designation to sulfur, the lone pair is always assigned the lowest priority (4). SAM, S -adenosylmethionine, is a biologically active sulfonium ion that we will learn about in Section 7.16. Locate all the stereogenic centers in SAM, and assign an R,S designation to each center.arrow_forwardNeed help identifying the right substituents in this question. The drop-down choices for the remaining three are the same.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
