
(a)
Interpretation:
Whether the given compound can undergo a Friedel–Crafts reaction, it is to be determined.
Concept introduction:
The Friedel–Crafts reaction is the electrophilic aromatic substitution reaction. In The Friedel–Crafts alkylation, the
Answer to Problem 23.51P
The given compound can undergo a Friedel–Crafts reaction as the aromatic ring is activated by two methyl groups.
Explanation of Solution
The given aromatic compound is:
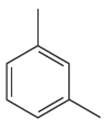
In this aromatic compound, the benzene ring has two methyl groups attached. The alkyl groups are electron donating inductively, thus increases the electron density around the ring and activates it. As the ring is electron rich and activated it can undergo Friedel–Crafts reaction.
It is determined that a given compound can undergo Friedel–Crafts reaction based on the activation of aromatic ring.
(b)
Interpretation:
Whether the given compound can undergo a Friedel–Crafts reaction, it is to be determined.
Concept introduction:
The Friedel–Crafts reaction is the electrophilic aromatic substitution reaction. In The Friedel–Crafts alkylation, the aromatic compound reacts with alkyl halide in presence of
Answer to Problem 23.51P
The given compound cannot undergo a Friedel–Crafts reaction as the aromatic ring is deactivated by two acetyl groups.
Explanation of Solution
The given aromatic compound is:
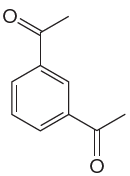
In this aromatic compound, the benzene ring has two acetyl (carbonyl) groups attached. The carbonyl groups have electron withdrawing resonance effect, thus decreases the electron density around the ring and deactivates it. As the ring is electron poor and deactivated it cannot undergo Friedel–Crafts reaction.
It is determined that a given compound cannot undergo Friedel–Crafts reaction based on the deactivation of aromatic ring.
(c)
Interpretation:
Whether the given compound can undergo a Friedel–Crafts reaction, it is to be determined.
Concept introduction:
The Friedel–Crafts reaction is the electrophilic aromatic substitution reaction. In The Friedel–Crafts alkylation, the aromatic compound reacts with alkyl halide in presence of
Answer to Problem 23.51P
The given compound cannot undergo a Friedel–Crafts reaction as the aromatic ring is deactivated by nitrile groups.
Explanation of Solution
The given aromatic compound is:
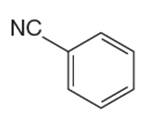
In this aromatic compound, the benzene ring has nitrile group attached. The nitrile group have electron withdrawing resonance effect, thus decreases the electron density around the ring and deactivates it. As the ring is electron poor and deactivated it cannot undergo Friedel–Crafts reaction.
It is determined that a given compound cannot undergo Friedel–Crafts reaction based on the deactivation of aromatic ring.
(d)
Interpretation:
Whether the given compound can undergo a Friedel–Crafts reaction, it is to be determined.
Concept introduction:
The Friedel–Crafts reaction is the electrophilic aromatic substitution reaction. In The Friedel–Crafts alkylation, the aromatic compound reacts with alkyl halide in presence of
Answer to Problem 23.51P
The given compound cannot undergo a Friedel–Crafts reaction as the aromatic ring is deactivated by
Explanation of Solution
The given aromatic compound is:
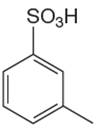
In this aromatic compound, the benzene ring has
It is determined that a given compound cannot undergo Friedel–Crafts reaction based on the deactivation of aromatic ring.
(e)
Interpretation:
Whether the given compound can undergo a Friedel–Crafts reaction, it is to be determined.
Concept introduction:
The Friedel–Crafts reaction is the electrophilic aromatic substitution reaction. In The Friedel–Crafts alkylation, the aromatic compound reacts with alkyl halide in presence of
Answer to Problem 23.51P
The given compound cannot undergo a Friedel–Crafts reaction as the aromatic ring is deactivated by
Explanation of Solution
The given aromatic compound is:
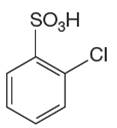
In this aromatic compound, the benzene ring has
It is determined that a given compound cannot undergo Friedel–Crafts reaction based on the deactivation of aromatic ring.
(f)
Interpretation:
Whether the given compound can undergo a Friedel–Crafts reaction, it is to be determined.
Concept introduction:
The Friedel–Crafts reaction is the electrophilic aromatic substitution reaction. In The Friedel–Crafts alkylation, the aromatic compound reacts with alkyl halide in presence of
Answer to Problem 23.51P
The given compound can undergo a Friedel–Crafts reaction as the aromatic ring is activated by one methyl and one methoxy groups.
Explanation of Solution
The given aromatic compound is:
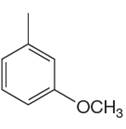
In this aromatic compound, the benzene ring has one methyl and one methoxy groups attached. The alkyl groups are electron donating inductively and methoxy group has electron donating resonance effect, thus increases the electron density around the ring and activates it. As the ring is electron rich and activated it can undergo Friedel–Crafts reaction.
It is determined that a given compound can undergo Friedel–Crafts reaction based on the activation of aromatic ring.
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





