
(a)
Interpretation:
The increasing order of
Concept introduction:
The nucleophilic substitution reactions are the reactions in which one nucleophile is substituted by another nucleophile. These reactions depend upon the nucleophilicity and concentration of the nucleophile. It is of two types,
The
The
Answer to Problem 18.1P
The given compounds with the increasing order of
Explanation of Solution
The structure of given compounds is shown below
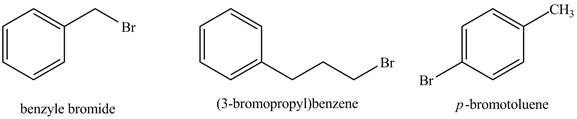
Figure 1
The
In
Therefore, the given compounds with the increasing order of
The increasing order of
(b)
Interpretation:
The increasing order of
Concept introduction:
The nucleophilic substitution reactions are the reactions in which one nucleophile is substituted by another nucleophile. These reactions depend upon the nucleophilicity and concentration of the nucleophile. It is of two types,
The
The
Answer to Problem 18.1P
The given compounds with the increasing order of
Explanation of Solution
The structure of given compounds is shown below.
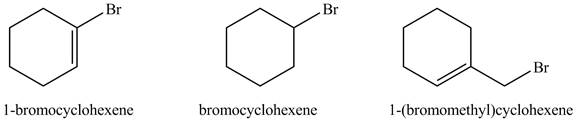
Figure 2
The
In
The increasing order of
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry
- 1. At what position and on what ring would you expect the following substances to undergo electrophilic substitution? (b) CH3 Br lel CH3 2. Rank the compounds in each group according to their reactivity toward electrophilic substitution. (a) Chlorobenzene, o-dichlorobenzene, benzene (b) p-Bromonitrobenzene, nitrobenzene, phenol (c) Fluorobenzene, benzaldehyde, 0-xylene (d) Benzonitrile, p-methylbenzonitrile, p-methoxybenzonitrilearrow_forwardPropose mechanisms and draw reaction-energy diagrams for the following reactions. Pay particular attention to the structures of any transition states and intermediates. Compare the reaction-energy diagrams for the two reactions, and explainthe differences.(a) 2-Bromo-2-methylbutane reacts with sodium methoxide in methanol to give 2-methylbut-2-ene (among other products).(b) 2-Bromo-2-methylbutane reacts in boiling methanol to give 2-methylbut-2-ene (among other products)arrow_forwardThe reaction of 1-bromopropane and sodium hydroxide in ethanol occurs by an SN2mechanism. What happens to the rate of this reaction under the following conditions?(a) The concentration of NaOH is doubled.(b) The concentrations of both NaOH and 1-bromopropane are doubled.(c) The volume of the solution in which the reaction is carried out is doubled.arrow_forward
- Propose a mechanism for the reaction of(a) 1-methylcyclohexanol with HBr to form 1-bromo-1-methylcyclohexane.(b) 2-cyclohexylethanol with HBr to form 1-bromo-2-cyclohexylethane.arrow_forwardPlease give the main substitution product for each of the following reactions, and indicate the dominant mechanism: (a) 1-bromopropane + NaOCH3 → (b) 3-bromo-3-methylpentane + NaOC2H5 →arrow_forwardH3C N- H₂NNH₂ H⭑ CH3 H3C IN CH3 Hydrazine reacts with 2,4-pentanedione to yield 3,5-dimethylpyrazole. Including protonations and deprotonations, the reaction takes 12 steps. Write out the mechanism on a sheet of paper and then draw the structure of the product of step 6. • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • In an elimination step, include the structure of the leaving group, but draw it in its own sketcher. Separate structures with + signs from the drop-down menu. ? Sn th Previous Nextarrow_forward
- Treatment of propadiene (an allene) with hydrogen bromide produces 2-bromopropene as the major product. This suggests that the more stable carbocation intermediate is produced by the addition of a proton to Br HBr. H2C=C=CH, H3C CH2 a terminal carbon rather than to the central carbon. Propadiene 2-Bromopropene (a) Draw both carbocation intermediates that can be produced by the addition of a proton to the allene. (b) Explain the relative stabilities of those intermediates. Hint: Draw the orbital picture of the intermediates and consider whether the CH, groups in propadiene are in the same plane.arrow_forwardWhen propenal is treated with sodium acetylide, a product is formed whose IR spectrum exhibits a broad absorption between 3200 and 3600 cm¯1, but shows no absorption near 1700 cm-1. (a) Draw the structure of the product. (b) Argue whether the nucleophile adds reversibly or irreversibly to the carbonyl group. CH HC || CH2 1. HC=CNa ? 2. NH,CIarrow_forwardTreatment of cis-4-bromocyclohexanol with HO− affords compound Aand cyclohex-3-en-1-ol. Treatment of trans-4-bromocyclohexanol under the same conditions forms compound B and cyclohex-3-en-1-ol. A and Bcontain different functional groups and are not isomers of each other.Propose structures for A and B and offer an explanation for theirformation.arrow_forward
