
Concept explainers
Oxidation of citronellol, a constituent of rose and geranium oils, with PCC in the presence of added
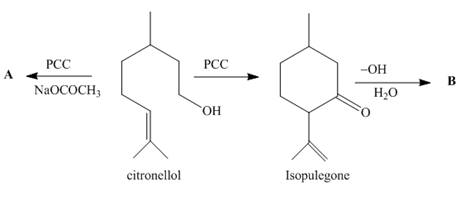
(a) Identify the structures of A and B
(b) Draw a mechanism for the conversion of citronellol to isopulegone.
(c) Draw a mechanism for the conversion of isopulegone to
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry by OpenStax (2015-05-04)
Chemistry For Changing Times (14th Edition)
Organic Chemistry (8th Edition)
Chemistry: Structure and Properties
- Compound I (C11H14O2) is insoluble in water, aqueous acid, and aqueous NaHCO3, but dissolves readily in 10% Na2CO3 and 10% NaOH. When these alkaline solutions are acidified with 10% HCl, compound I is recovered unchanged. Given this information and its 1H-NMR spectrum, deduce the structure of compound I.arrow_forwardAddition of m-xylene to the strongly acidic solvent HF/SbF5 at 45C gives a new species, which shows 1H-NMR resonances at 2.88 (3H), 3.00 (3H), 4.67 (2H), 7.93 (1H), 7.83 (1H), and 8.68 (1H). Assign a structure to the species giving this spectrum.arrow_forwardTreatment of benzoic acid (C6H5CO2H) with NaOH followed by 1-iodo-3methylbutane forms H. H has a molecular ion at 192 and IR absorptions at 3064, 3035, 2960−2872, and 1721 cm−1. Propose a structure for H.arrow_forward
- Treatment of the bicyclic chloride A with lithium in tetrahydrofuran (THF) gave the monocyclic product B, C,H,Li. In deuterated THF, B exhibited a single proton NMR signal at 6.72 ppm and a single 13 C-NMR signal at about 110 ppm. Treatment of B with tetraethylammonium chloride gave a white solid in which N(C2H5)4 replaces Li. Draw a structure for the lithium derivative B. A Li/THF -CI CgHgLi (C2H5)4N CI C9H9N(C2H5)4 B Draw cations and anions in separate sketchers. ⚫ Separate structures with + signs from the drop-down menu.arrow_forwardAn unknown compound C3H2NCl shows moderately strong IR absorptions around 1650 cm-1 and 2200 cm-1. Its NMR spectrum consists of two doublets (J = 14 Hz) at δ 5.9 and δ 7.1. Propose a structure consistent with this data?arrow_forwardTreatment of 2-methylpropanenitrile [(CH3)2CHCN] with CH3CH2CH2MgBr, followed by aqueous acid, affords compound V, which has molecular formula C7H14O. V has a strong absorption in its IR spectrum at 1713 cm−1, and gives the following 1H NMR data: 0.91 (triplet, 3 H), 1.09 (doublet, 6 H), 1.6 (multiplet, 2 H), 2.43 (triplet, 2 H), and 2.60 (septet, 1 H) ppm. What is the structure of V? We will learn about this reaction in Chapter 20.arrow_forward
- Compound B of molecular formula C9H19N shows a noteworthy infrared absorption at 3300 cm-1. Its 1H-NMR spectrum shows three singlets – δ 1.0 (6H), 1.1 (12H), 1.4 (1H) ppm. Its 13C-NMR spectrum has four signals – δ 25, 28, 41, 64 ppm. Suggest a structure for this compound. Please show work.arrow_forwardThere are several isomeric alcohols and ethers of molecular formula C5H12O. Two of these exhibit the following 1H-NMR spectra. Propose a structure for each of the isomers. Isomer A: δ = 0.92 (t, 7.8 Hz, 3 H), 1.20 (s, 6H), 1.49 (q, 7.8 Hz, 2H), 1.85 (s, 1H) ppm Isomer B: δ = 1.19 (s, 9 H), 3.21 (s, 3H) ppmarrow_forwardReaction of (CH3)3CCHO with (C6H5)3P=C(CH3)OCH3, followed by treatment with aqueous acid, affords R (C7H14O). R has a strong absorption in its IR spectrum at 1717 cm−1 and three singlets in its 1H NMR spectrum at 1.02 (9 H), 2.13 (3 H), and 2.33 (2 H) ppm. What is the structure of R? We will learn about this reaction in Chapter 18.arrow_forward
- 22. A compound with the molecular formula C8H8O produces an IR spectrum with signals at 3063, 1686, and 1646 cm-1. The 1H NMR spectrum of this compound exhibits a singlet at 2.6ppm(l=3H), and a multiplet at 7.5(l= 5H). Draw the structure and give the common name of this compound . Show the correlations between the structure and spectra.arrow_forwardProvide a structure for the compound with molecular formula C5H10O and with the following spectroscopic data.IR: 1720 cm−11H NMR: 0.9δ (triplet, I=3H), 1.7δ (sextet, I=2H), 2.1δ (singlet, I=3H), 2.4δ (triplet, I=2H)arrow_forward3-Chlorocyclopropene, on treatment with AgBF4, gives a precipitate of AgCl and a stable solution of a product that shows a single 1H NMR absorption at 11.04 δ. What is a likely structure for the products, and what is its relation to HĂ¼ckel’s rule?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

