
(a)
Interpretation:
The structure of the molecule that corresponds to the given IUPAC name is to be drawn.
Concept introduction:
The rules for naming acid anhydrides are derived from the fact that an acid anhydride can be produced from two carboxylic acids in the dehydration reaction as shown below:
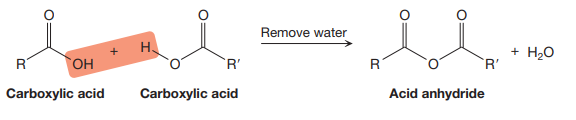
If the two R and R’ groups attached to the acid anhydride are the same, then the anhydride is symmetrical, but if they are different, the acid anhydride is unsymmetrical.
Symmetrical anhydrides are named according to the general form alkanoic anhydride in which the alkanoic portion corresponds to the specific
Unsymmetrical anhydrides are named according to the general form alkanoic alkanoic anhydride, where each alkanoic portion corresponds to different carboxylic acids that would be required to produce the anhydride. The two carboxylic acids follow the alphabetical order.
(b)
Interpretation:
The structure of the molecule that corresponds to the given IUPAC name is to be drawn.
Concept introduction:
The rules for naming acid anhydrides are derived from the fact that an acid anhydride can be produced from two carboxylic acids in the dehydration reaction as shown below:

If the two R and R’ groups attached to the acid anhydride are the same, then the anhydride is symmetrical, but if they are different, the acid anhydride is unsymmetrical.
Symmetrical anhydrides are named according to general form alkanoic anhydride where the alkanoic portion corresponds to the specific carboxylic acid that could undergo dehydration to produce the anhydride.
Unsymmetrical anhydrides are named according to the general form alkanoic alkanoic anhydride, where each alkanoic portion corresponds to different carboxylic acids that would be required to produce the anhydride. The two carboxylic acids follow the alphabetical order.
(c)
Interpretation:
The structure of the molecule is to be drawn that corresponds to the given IUPAC name.
Concept introduction:
The rules for naming acid anhydrides are derived from the fact that an acid anhydride can be produced from two carboxylic acids in the dehydration reaction as shown below:

If the two R and R’ groups attached to the acid anhydride are same, then the anhydride is symmetrical, but if they are different, the acid anhydride is unsymmetrical.
Symmetrical anhydride are named according to general form alkanoic anhydride where the alkanoic portion corresponds to the specific carboxylic acid that could undergo dehydration to produce the anhydride.
Unsymmetrical anhydrides are named according to the general form alkanoic alkanoic anhydride, where each alkanoic portion corresponds to different carboxylic acids that would be required to produce the anhydride. The two carboxylic acids follow the alphabetical order.
Substituents attached to the carbon chain of any carboxylic acid portion are written as prefix in the IUPAC name.
(d)
Interpretation:
The structure of the molecule is to be drawn that corresponds to the given IUPAC name.
Concept introduction:
The rules for naming acid anhydrides are derived from the fact that an acid anhydride can be produced from two carboxylic acids in the dehydration reaction as shown below:

If the two R and R’ groups attached to the acid anhydride are same, then the anhydride is symmetrical, but if they are different, the acid anhydride is unsymmetrical.
Symmetrical anhydride are named according to general form alkanoic anhydride where the alkanoic portion corresponds to the specific carboxylic acid that could undergo dehydration to produce the anhydride.
Unsymmetrical anhydrides are named according to the general form alkanoic alkanoic anhydride, where each alkanoic portion corresponds to different carboxylic acids that would be required to produce the anhydride. The two carboxylic acids follow the alphabetical order.
Substituents attached to the carbon chain of any carboxylic acid portion are written as prefix in the IUPAC name.
Want to see the full answer?
Check out a sample textbook solution
Chapter F Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Identify the major organic product(s) of the following reaction. x A. B. 1) KOH D. ه (2 مسلسل موسلی yasarrow_forwardA- What is the definition of acidity? B- Compare the acidity of ammonia and its aliphatic derivatives with of acidity pyrrole C- Melting points of pyrrole is higher than melting point of 1-methyl pyrrole explain your answer D- Why does pyrrole prefer electrophilic substitution reactions? E- Why Pyrrole is considered to be an aromatic compound ? F- Explain the Reimer-Tiemann reaction mechanism of heterocyclic compounds? G- Why pyridine is a weak base? explain your answer? H- Pyridine can react with electrophiles, electrophilic substitution ? explain your answer ? G- Explain the Diels–Alder reaction mechanism of heterocyclic compounds? I- From a-haloketone how can you prepared flowing compounds : Imidazole Oxazole Thiazole J- compared the acidity and basicity of Pyrazole and Imidazole with Pyrrole and Pyridine ?arrow_forward(6) Draw the structure for each solvent and explain why it is polar protic or aprotic. a. DMSO b. THF c. Methylaminearrow_forward
- Aldehydes and ketones give additon reaction with a.phenylhydrazine b..hydrazine c.HCN d. All of the abovearrow_forward3. Devise a synthesis of each ester from benzene, organic alcohols, and any other needed inorganic reagents. 4. Provide the structures of a. propanoic anhydride b. o-bromobenzoyl chloride NH, Ọarrow_forward2. Complete the following electrophilic aromatic substitution reactions for the monosubstituted benzenes, Providing major product(s) only. (Hint: start with identifying the effect of substituent, then figuring out the structure of product) b. C. d. 1. 9₁ ,NO Br CO₂H B1₂ FoBra SO, H₂SO4 EICI AICI Cl₂ FeCl HNO3 H₂SO4 CI Br₂ CH₂COOH 1. SnCl, HyO* 2. H₂O AICI3arrow_forward
- - Draw the structural formula of compounds K, I, L and J.- Reagents for M and N.arrow_forward10. Provide unambiguous structural formulas for reaction products A, B, and C. Br 1) I (2 eq.) 2) B(OMe)3 Pd(PPhala Grubbs cat. Barrow_forward1. a. 4-methoxybenzoic acid is less or more polar than 4-methoxyacetophenone? explain why (WITHOUT DRAWINGS) b. 3'-chloro-4'-methoxyacetophenone is less or more polar than 4-methoxyacetophenone? explain why (WITHOUT DRAWINGS) 2. a. 4-methoxybenzoic acid has a higher melting point than 4-methoxyacetophenone. explain why? (WITHOUT DRAWINGS) b. 3'-chloro-4'-methoxyacetophenone has a higher melting point than 4-methoxyacetophenone. explain why? (WITHOUT DRAWINGS)arrow_forward
- 2. Carbomer is a polycarboxylic acid. Draw a titration curve, a axis with pH against the added amountstrong base, for the monoic acid (e.g. acetic acid) and polyic acid (Carbomer) and explain the difference.arrow_forwardGive a clear explanation handwritten answer....give the all missing reagent to Complete the reactions....arrow_forward28. The following compounds undergo electrophilic aromatic substitution EXCEPT A. I and II B. III and IV C. II and III D. III I. II. 29. Which alkene is the most stable? A. I B. II C. III D. IV III. II. III. IV. IV. 30. In the hydrohalogenation reaction of 1-phenylpropene, only 1-chloro-1-phenylpropane is obtained because A. a more stable carbonium ion is generated from this product. B. a more stable radical intermediate is generated from this product. C. a more stable benzene ring is generated from this product. D. a less stable transition state is generated from this product.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





