
Concept explainers
a)
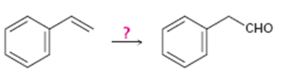
Interpretation:
How to carry out the conversion shown is to be stated.
Concept introduction:
Terminal
To state:
How to carry out the conversion shown.
b)
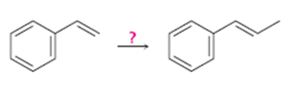
Interpretation:
How to carry out the conversion shown is to be stated.
Concept introduction:
The alkene is converted in to a dibromo derivative by treating with Br2 in the presence of CH2Cl2. The dibromo derivative is then treated with two equivalents of KOH when dehydrohalogenation takes place yield a terminal
To state:
How to carry out the conversion shown.
Trending nowThis is a popular solution!

Chapter 9 Solutions
ORGANIC CHEMISTRY W/OWL
- Show how you might bring about the following conversions. For any conversion involving more than one step, show intermediate compoundarrow_forwardCH3 to Br- -H + Nal • product; Sy2 product of the reaction is : Аcetone H- -CH3 CH2 – CH3 CH3 CH3 CH3 CH3 it it -H H- -CH3 (d) -CH3 -H- (а) H• (Ъ) H- -CH3 -CH3 (с) H- CH -H- ČH2 – CH3 ČH2 – CH3 CH2 – CH3 CH2- CH3 2.arrow_forwardWhich structures have the correct IUPAC name? CH,OH носн, `CH, cis-1,2-dimethoxycyclohexane R-2-methoxy-1-propanol II он H,C HC-OCH, H,C ´ OH 2-methoxypropane III trans-1,3-cyclohexanediol iv O A. I, II O B. II, IV O C. II, II O D. I, IVarrow_forward
- Rank the molecules A to D shown below by decreasing reactivity towards lodo cyclohexane. H3C-CH,OH F,C-CH,OH H,C-CH,NH, H3C-N-CH, A в D O 1.C>D>A>B O 2.B> A> C> D O 3. A> C> B > D O 4. A> B>D>Carrow_forward3. Complete the road map below providing the reagent and or products in the boxes as indicated. Please note that each box may contain more than one reagent/step. Br₂ 1. Xs NaNHz 2. H₂O C5H10 HOarrow_forward10.35 Show how you might bring about the following conversions. For any conversion involv- ing more than one step, show each intermediate compound. Toy thalgy LOH (b) HOED HO lemburan, and ignonte dass 101 od 15grote schmal au OH 0-OH OH (a) (c) (e) Лон (i) ОН — 1000 110 10 112 115 HO 10 HO HO Yo OH bir rico sd send ogniz sili CH₂ dilipp besc-sain CH, JESH IH (6 → (b) (6 (d) H1) (d) CH₂Cl O + OH -> rognous siit [del murgiliups (los HO HO HO 00 CHO CH₂OH OH 191 1992 hiwted-Ito (h) 5.0 7 OH TOHO HD 10 2H0D) Ö De din inacted to OH isod 1811 (3) 184 10 odot LOH 210 750 11000-16) H 51 to enarrow_forward
- Name the following organic compound: CH3 S CH CH3 CH 0 CH, CH, 3-methylsulfide-2-ethoxy butane 2-ethoxy-3-methylthio butane O 3-ethoxy-2-methylthio butane O 2-methylsulfide-3-ethoxy butane O 2-ethoxy-3-methylsulfide butanearrow_forwardConvert neopentane (molecule A) to 4,4-dimethylpentanal (molecle B):arrow_forwardIUPAC name of the following compound is: OC-H5 OCH3 OCH3 A 1-Ethoxy-2,2-dimethylcyclohexane (B 1-Ethoxy-2,2-dimethoxycyclohexane C 2-ethoxy-1,1-dimethoxybenzene D 2-Ethoxy-1,1-dimethoxycyclohexanearrow_forward
- Starting with cyclohexane and using any other carbon compounds containing 3 carbons or less, synthesize two of the following compounds. These will be multi-step processes. CH a) H,C b) CH, HO c) HO.arrow_forwardplease show a step by step mechanism R1 82% yield (Ref: Taylor) (3cis) R1: OCOMe, R2: H (3trans) R1: H, R2: OCOME 1. 40% NAOH (aq) 2. CH;Cl2 3. CHBR3 4. 40°C, 24h Br Brarrow_forwardA Assign E or Z to the following alkenes... H3C CH3 # 1: #2: ( 1 = E, 2 = 2 ( 1 = Z, 2 = E O 1 & 2 are Z O1 & 2 are E H2C-CH HOH C Н.С H3CO C=C CH₂CH(CH3)2 ОН OCH 3 CH3arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

