
Concept explainers
(a)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an
Answer to Problem 9.66P
The product formed by the treatment of ethylene oxide with the given reagent is,
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
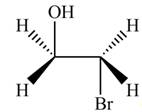
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
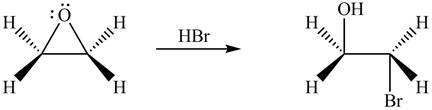
Figure 1
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 1.
(b)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.66P
The product formed by the treatment of ethylene oxide with the given reagent is,
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
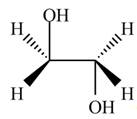
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
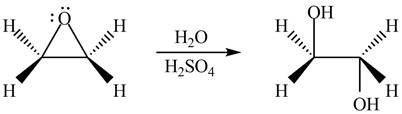
Figure 2
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 2.
(c)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.66P
The product formed by the treatment of ethylene oxide with the given reagent is,
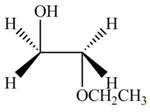
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
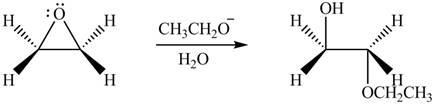
Figure 3
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 3.
(d)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.66P
The product formed by the treatment of ethylene oxide with the given reagent is,
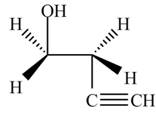
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
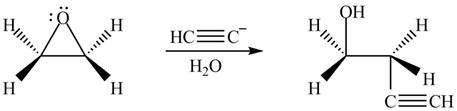
Figure 4
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 4.
(e)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.66P
The product formed by the treatment of ethylene oxide with the given reagent is,
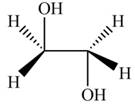
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
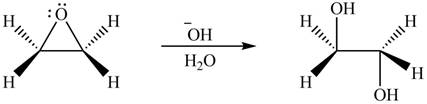
Figure 5
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 5.
(f)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.66P
The product formed by the treatment of ethylene oxide with the given reagent is,
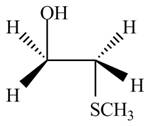
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
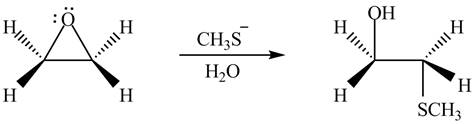
Figure 6
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 6.
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry
- 61. Give the products of the following acid-base reactions and indicate whether reactants or products are favored at equilibrium. (Use the pk, values that are given in Section 2.3.) CH,NH; = a. CH,COH + CH;0 b. CH,CH,OH + NH, c. CH;COH + d. CH;CH,OH + HCI =arrow_forwardSelect the correct product for the following reaction. CI O a. O b. OC O d. Oe. O h CI HO HO H 0= O 1. DIBAL 2. H3O+arrow_forward16. Predict the products of the following reactions. ( a. Br 1. Mg/ether 2. CO2 3. H,O* b. NH d. HO Br CN 1. LDA e. 2. CH;CH,Iarrow_forward
- Draw the products of each proton transfer reaction. a. CCl,cO,H OCH3 c. CH3NH, HCI b. Н-С-с-н H: d. CH;CH,OH H2SO4arrow_forwardWhat is the product of this reaction? HO A. B. O A OB O C OD C OH H* cat. C. D.arrow_forward4. Please write the type of reaction each equation represents (elimination, addition, rearrangement, substitution). Write your answers beneath each reaction. HNE Pd catatlyst HyC NaCN NE acid cat NC "OH CH Lewis acid Me base UV ightarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





