
What
results.
a. 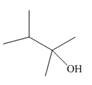 b.
b. 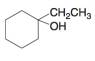 c.
c. 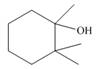 d.
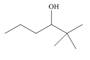
d.
(a)
Interpretation: The alkenes formed by the treatment of given alcohol with
Concept introduction: Alcohols undergo dehydration reaction in the presence of strong acids like
Answer to Problem 9.48P
The alkenes formed by the treatment of given alcohol with
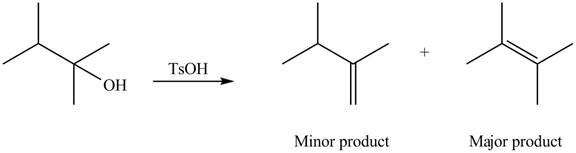
Explanation of Solution
The given alcohol contains two
Alcohols undergo dehydration reaction in the presence of strong acids like
Thus, the alkenes formed by the treatment of given alcohol with
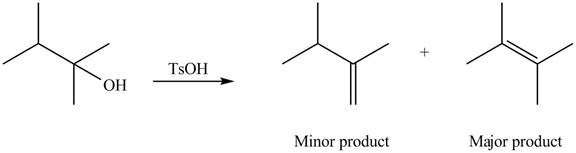
Figure 1
The alkenes formed by the treatment of given alcohol with
(b)
Interpretation: The alkenes formed by the treatment of given alcohol with
Concept introduction: Alcohols undergo dehydration reaction in the presence of strong acids like
Answer to Problem 9.48P
The alkenes formed by the treatment of given alcohol with
Explanation of Solution
The given alcohol contains two
Alcohols undergo dehydration reaction in the presence of strong acids like
Thus, the alkenes formed by the treatment of given alcohol with

Figure 2
The alkenes formed by the treatment of given alcohol with
(c)
Interpretation: The alkenes formed by the treatment of given alcohol with
Concept introduction: Alcohols undergo dehydration reaction in the presence of strong acids like
Answer to Problem 9.48P
The alkenes formed by the treatment of given alcohol with
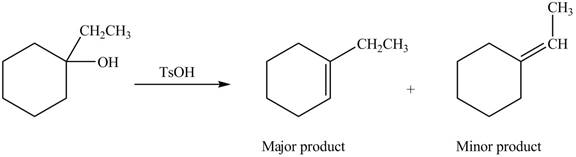
Explanation of Solution
The given alcohol contains three
Alcohols undergo dehydration reaction in the presence of strong acids like
Thus, the alkenes formed by the treatment of given alcohol with
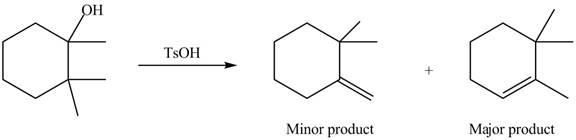
Figure 3
The alkenes formed by the treatment of given alcohol with
(d)
Interpretation: The alkenes formed by the treatment of given alcohol with
Concept introduction: Alcohols undergo dehydration reaction in the presence of strong acids like
Answer to Problem 9.48P
The alkene formed by the treatment of given alcohol with
Explanation of Solution
The given alcohol contains two
Alcohols undergo dehydration reaction in the presence of strong acids like
The alkenes formed by the treatment of given alcohol with
(e)
Interpretation: The alkenes formed by the treatment of given alcohol with
Concept introduction: Alcohols undergo dehydration reaction in the presence of strong acids like
Answer to Problem 9.48P
The alkenes formed by the treatment of given alcohol with
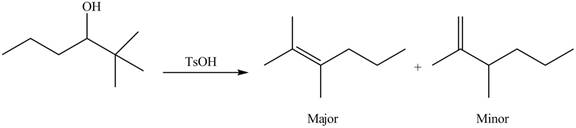
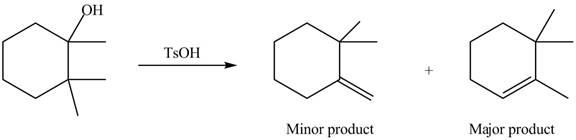
Explanation of Solution
The given alcohol contains two
Alcohols undergo dehydration reaction in the presence of strong acids like
Thus, the alkenes formed by the treatment of given alcohol with

Figure 4
The alkenes formed by the treatment of given alcohol with
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry
- Give the IUPAC name for each ketone. C-CHCH,CH3 b. a.arrow_forward16. Identify each compound as an a cohol, a phenol, or an ether. Classify any alcohols as primary (1"), secondary (2), or tertiary (3"). a. CH,CH,CH,OH CH,CHCH, b. CHO C. CH CHOCH, CH, d.arrow_forward18. Ketone reduction Dicyclohexyl ketone Reduce the ketone. 1. NaBH4, ethanol 2. H3O+ H OH Dicyclohexylmethanol (88%) (a 2° alcohol)arrow_forward
- Give the IUPAC name for each alcohol но сн CH,CHCCH,CH,CH3 (CH)½CHCH,CHCH,CH3 b. a. CH,CH,CH,OH HO. с. CH,CH,CH,CH, Draw the products formed when each alcohol is dehydrated with H2SO4. Use the Zaitsev rule to predict the major product when a mixture forms. OH он b. -CHCH,CH3 а. Он с. CH3CHCH2CH,CH;CH3arrow_forwardWhat alkenes are formed when each alcohol is dehydrated with TsOH? Label the major product when a mixture resultsarrow_forwardDraw the products of each reaction. CH3 a. CH3-C-CH,CH3 HCI HI OH HBr b. C. OHarrow_forward
- 1. Name the following alcohols according to IUPAC rules: a. NO₂ b. C. OH Name: CI OH OH Name: Name: OHarrow_forwardList the products of each alcohol reaction. CH3 a. CH,-C-OH CH, NazCrO b. CH3-CH-CH;-CH2-OH c. CH-CH-OH +HCI -arrow_forwardI. What products are formed when each alcohol is oxidized with K2Cr207? a. CH;CH,CH2CH;CH2OH ОН ОН H3C CH3 b. C.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





