
Concept explainers
(a)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an
Answer to Problem 9.64P
The product formed by the treatment of ethylene oxide with the given reagent is,
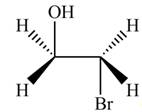
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
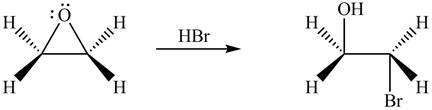
Figure 1
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 1.
(b)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.64P
The product formed by the treatment of ethylene oxide with the given reagent is,
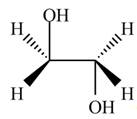
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
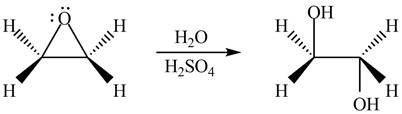
Figure 2
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 2.
(c)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.64P
The product formed by the treatment of ethylene oxide with the given reagent is,
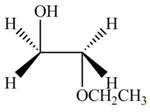
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
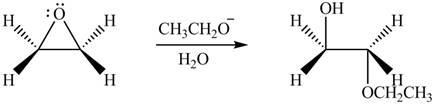
Figure 3
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 3.
(d)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.64P
The product formed by the treatment of ethylene oxide with the given reagent is,
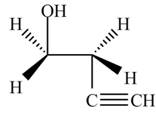
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
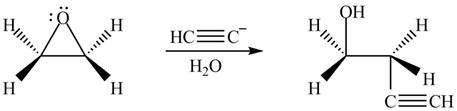
Figure 4
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 4.
(e)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.64P
The product formed by the treatment of ethylene oxide with the given reagent is,
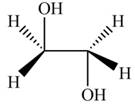
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
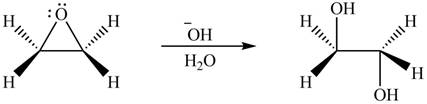
Figure 5
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 5.
(f)
Interpretation: The product formed by the treatment of ethylene oxide with the given reagent is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.64P
The product formed by the treatment of ethylene oxide with the given reagent is,
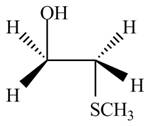
Explanation of Solution
The given reagent is
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product formed by the treatment of ethylene oxide with the given reagent is,
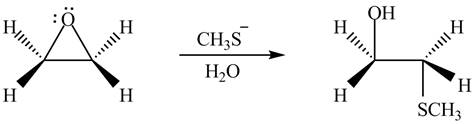
Figure 6
The product formed by the treatment of ethylene oxide with the given reagent is drawn in Figure 6.
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry
- #86arrow_forward4arrow_forwardQUESTION 2 The following is a proposed synthetic strategy that uses one starting alcohol in two separate single-step reactions (I and II) to make a pair of diene and dienophile which together undergo a Diels-Alder reaction to give a Diels-Alder product. The Diels-Alder product is subsequently converted to the final ether in a three-step synthesis (III, IV and V). Identify the reagent(s) from Table I required for each of those three sysnthese and place the number (A1, A2 reagent(s) in the given box right next to the corresponding step numbers (I to V) of the synthesis. DO NOT fill in any box with more than one number. (Note: This question is different from the previous synthesis question which allows two numbers for each box). You will be graded based on the number in each box. Do not fill in any single box with more than one number. Do not leave any box blank. B1, B2..., or C1, C2 ...) that represents the (I) diene OH (III, IV and V) Diesl-Alder product (II) dienophile Table I Number…arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





