
Organic Chemistry, Loose-leaf Version
8th Edition
ISBN: 9781305865549
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 9.33P
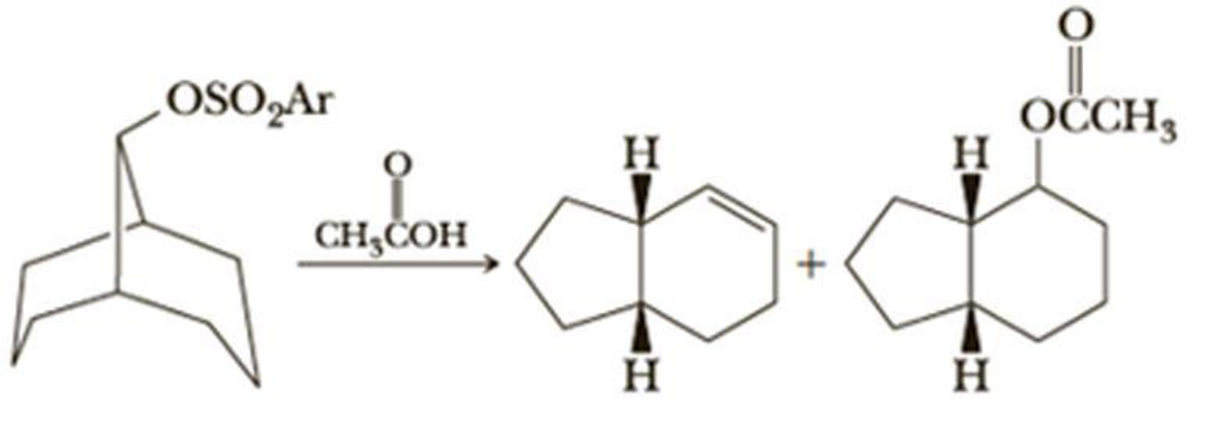
Solvolysis of the following bicyclic compound in acetic acid gives a mixture of products, two of which are shown. The leaving group is the anion of a sulfonic acid, ArSO3H. A sulfonic acid is a strong acid, and its anion, ArSO3–, is a weak base and a good leaving group. Propose a mechanism for this reaction.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Solvolysis of the following bicyclic compound in acetic acid gives a mixture of products,
two of which are shown. The leaving group is the anion of a sulfonic acid, ArSO,H.
A sulfonic acid is a strong acid, and its anion, ARSO, , is a weak base and a good leaving
group. Propose a mechanism for this reaction.
voso
OČCH,
CH,COH
Propose a mechanism for the following reaction. Draw out the each step of the reaction including
ALL intermediates.
CI
A
CO₂ Et
+
inte
NaO6t
EtOH
CTS
Eta₂c,
When the following compound undergoes solvolysis in ethanol, three products are obtained. Propose a mechanism to account for the formation ofthese products.
Chapter 9 Solutions
Organic Chemistry, Loose-leaf Version
Ch. 9.1 - Prob. 9.1PCh. 9.3 - Prob. 9.2PCh. 9.3 - Prob. 9.3PCh. 9.3 - Prob. 9.4PCh. 9.4 - Prob. 9.5PCh. 9.5 - Predict the -elimination product(s) formed when...Ch. 9.7 - Prob. 9.7PCh. 9.9 - Predict whether each reaction proceeds...Ch. 9.9 - Prob. AQCh. 9.9 - Prob. BQ
Ch. 9.9 - Prob. CQCh. 9.9 - Prob. DQCh. 9.10 - Prob. 9.9PCh. 9 - Prob. 9.10PCh. 9 - Prob. 9.11PCh. 9 - Prob. 9.12PCh. 9 - Prob. 9.13PCh. 9 - Prob. 9.14PCh. 9 - Prob. 9.15PCh. 9 - Treatment of 1-aminoadamantane, C10H17N, with...Ch. 9 - Prob. 9.17PCh. 9 - Prob. 9.18PCh. 9 - Prob. 9.19PCh. 9 - Prob. 9.20PCh. 9 - Attempts to prepare optically active iodides by...Ch. 9 - Draw a structural formula for the product of each...Ch. 9 - Prob. 9.23PCh. 9 - Alkenyl halides such as vinyl bromide, CH2=CHBr,...Ch. 9 - Prob. 9.25PCh. 9 - Prob. 9.26PCh. 9 - Prob. 9.27PCh. 9 - Show how you might synthesize the following...Ch. 9 - Prob. 9.29PCh. 9 - 1-Chloro-2-butene undergoes hydrolysis in warm...Ch. 9 - Prob. 9.31PCh. 9 - Prob. 9.32PCh. 9 - Solvolysis of the following bicyclic compound in...Ch. 9 - Which compound in each set undergoes more rapid...Ch. 9 - Prob. 9.35PCh. 9 - Prob. 9.36PCh. 9 - Draw structural formulas for the alkene(s) formed...Ch. 9 - Prob. 9.38PCh. 9 - Following are diastereomers (A) and (B) of...Ch. 9 - Prob. 9.40PCh. 9 - Elimination of HBr from 2-bromonorbornane gives...Ch. 9 - Which isomer of 1-bromo-3-isopropylcyclohexane...Ch. 9 - Prob. 9.43PCh. 9 - Prob. 9.44PCh. 9 - Draw a structural formula for the major organic...Ch. 9 - When cis-4-chlorocyclohexanol is treated with...Ch. 9 - Prob. 9.47PCh. 9 - The Williamson ether synthesis involves treatment...Ch. 9 - The following ethers can, in principle, be...Ch. 9 - Prob. 9.50PCh. 9 - Prob. 9.51PCh. 9 - Prob. 9.52PCh. 9 - Prob. 9.53PCh. 9 - Prob. 9.54PCh. 9 - Write the products of the following sequences of...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Prob. 9.59PCh. 9 - Another important pattern in organic synthesis is...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Prob. 9.62PCh. 9 - Prob. 9.63PCh. 9 - Prob. 9.64P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When treated with base, the following compound undergoes an intramolecular aldol reaction and dehydration to give a product containing a ring. H Propose a structure for this product. base C4H6O + HOarrow_forwardReaction of phenol with acetone in the presence of an acid catalyst gives a compound known as bisphenol A, which is used in the production of epoxy and polycarbonate resins Propose a mechanism for the formation of bisphenol A. OH H;PO, + H,O НО HO Phenol Acetone Bisphenol Aarrow_forwardPayalarrow_forward
- When a methyl ester is hydrolyzed under acidic conditions in H,180, the 180 isotope ends up in the carboxylic acid. When a tert-butyl ester is hydrolyzed under the same conditions, the labeled oxygen ends up in the alcohol product. (a) Propose mechanisms to account for these observations. (b) Explain why each ester undergoes the respective mechanism. CH3 + Но-СНЗ 18 + H180 H ОНarrow_forwardA carboxylic acid is formed when an a-haloketone reacts with hydroxide ion. This reaction is called a Favorskii reaction. Propose a mechanism for thefollowing Favorskii reaction. (Hint: In the first step, HO- removes a proton from the a-carbon that is not bonded to Br; a three-membered ring is formedin the second step; and HO- is a nucleophile in the third step.)arrow_forwardVoltooi die volgende reaksie en stel 'h meganisme voor. Complete the following reaction and propose a mechanism. 1) CO2, P, A 2) H20, H*arrow_forward
- Addition of tert-butylbenzene to the strongly acidic solvent HF/SbF5 followed by aqueous workup gives benzene. Propose a mechanism for this dealkylation reaction. What is the other product of the reaction?arrow_forwardComplete each reaction scheme below with the starting material required to give the product shown as the only major product following the indicated sequence of reactions. Each individual reaction in the sequence must serve a purpose and must result in a product that is different from the corresponding starting material. 1) i) LIAIH, ii) H30* 2) TSCI, pyridine OTsarrow_forwardEthylene oxide is the starting material for the synthesis of 1,4-dioxane. Propose a mechanism for each step in this synthesis.arrow_forward
- Nonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardWhen the alcohol below is treated with POCI3 and pyridine, the expected elimination product is formed. However, when the same alcohol is treated with Η2SΟ4, the elimination product is 1, 2-dimethyl- cyclopentene. Propose a mechanism for each pathway to account for these differences.arrow_forwardA step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License