
(a)
Interpretation:
The most stable carbocation structural formula of given molecular formula has to be drawn.
Concept Introduction:
The most stable carbocation structural formula:
The most stable structural arrangement of atoms in a carbocation molecule is known as most stable carbocation structural formula.
The highly alkyl substituted carbocation is more stable
Hence, the stability of carbocation is,
(a)
Answer to Problem 9.10P
The most stable carbocation structural formula of given molecular formula is,
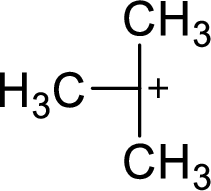
Explanation of Solution
The highly alkyl substituted carbocation is more stable. Hence, the most stable carbocation structural formula of given molecular formula is,
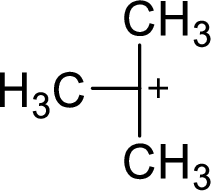
(b)
Interpretation:
The most stable carbocation structural formula of given molecular formula has to be drawn.
Concept Introduction:
The most stable carbocation structural formula:
The most stable structural arrangement of atoms in a carbocation molecule is known as most stable carbocation structural formula.
The highly alkyl substituted carbocation is more stable
Hence, the stability of carbocation is,
(b)
Answer to Problem 9.10P
The most stable carbocation structural formula of given molecular formula is,
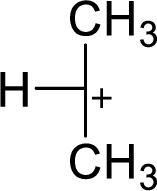
Explanation of Solution
The highly alkyl substituted carbocation is more stable. Hence, the most stable carbocation structural formula of given molecular formula is,
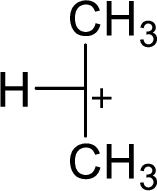
(c)
Interpretation:
The most stable carbocation structural formula of given molecular formula has to be drawn.
Concept Introduction:
The most stable carbocation structural formula:
The most stable structural arrangement of atoms in a carbocation molecule is known as most stable carbocation structural formula.
The highly alkyl substituted carbocation is more stable
Hence, the stability of carbocation is,
(c)
Answer to Problem 9.10P
The most stable carbocation structural formula of given molecular formula is,
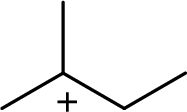
Explanation of Solution
The highly alkyl substituted carbocation is more stable. Hence, the most stable carbocation structural formula of given molecular formula is,
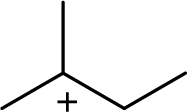
(d)
Interpretation:
The most stable carbocation structural formula of given molecular formula has to be drawn.
Concept Introduction:
The most stable carbocation structural formula:
The most stable structural arrangement of atoms in a carbocation molecule is known as most stable carbocation structural formula.
The highly alkyl substituted carbocation is more stable
Hence, the stability of carbocation is,
(d)
Answer to Problem 9.10P
The most stable carbocation structural formula of given molecular formula is,

Explanation of Solution
The highly alkyl substituted carbocation is more stable. Hence, the most stable carbocation structural formula of given molecular formula is,

Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry, Loose-leaf Version
- (a) (b) HO N N-H Catalytic H+ (-H₂O) ? (c) NH2 HO N (d)arrow_forward1.What is the product of the reaction between 1,3-dibutene and bromoethene? (A) No reaction occurs (B) 4-bromocyclohexene (C) 3-bromocyclohexene (D) 3-bromocyclopentene 2. Cyclohexene undergoes hydrobromination. Which of these is a possible product? (A) Bromocyclohexane (B) All of these (C) Trans 1,2-dibromocyclohexane (D) Cis 1,2-dibromocyclohexane 3. A hydrocarbon molecule is saturated if the molecule contains (A) Single covalent bonds, only (B) A double covalent bond, only (C) A triple covalent bond (D) Single and double covalent bondsarrow_forward(b) Identify the carbon atoms in the following molecules as primary (1°), secondary (2°), tertiary (3°), or quaternary (4°). (a) CH3 (Б) CH3CHCH3 (c) CH3 CH3 CH3CHCH2CH2CH3 CH3CH2CHCH2CH3 CH3CHCH2CCH3 CH3arrow_forward
- (−)-Menthol is the most stable stereoisomer of 2-isopropyl-5-methylcyclohexanol and has the R configuration at the hydroxyl-substituted carbon. (a) Draw the preferred conformation of (−)-menthol. (b) (+)-Isomenthol has the same constitution as (−)-menthol. The configurations at C-1 and C-2 of (+)-isomenthol are the opposite of the corresponding chirality centers of (−)-menthol. Write the preferred conformation of (+)-isomenthol.arrow_forward5.47 Show how to convert methylenecyclohexane into each of these compounds. -CH2OH (a) OH (b) ECH2 CH3 (c) Methylenecyclohexanearrow_forward2. Draw the two chair conformations of cis-1-ethyl-4-methylcyclohexane, and determine which of the two conformations is more stable, and why. Remember to draw in all hydrogens. (2) ba195ta 1o (3) barrow_forward
