
Chemistry (Instructor's)
10th Edition
ISBN: 9781305957787
Author: ZUMDAHL
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 88CWP
Pelargondin is the molecule responsible for the red color of the geranium flower. It also contributes to the color of ripe strawberries and raspberries. The structure of pelargondin is:
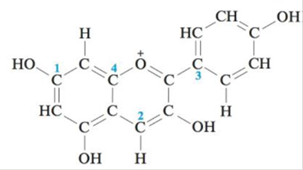
How many σ and π bonds exist in pelargondin? What is the hybridization of the carbon atoms marked 1–4?
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 9 Solutions
Chemistry (Instructor's)
Ch. 9 - Why do we hybtidize atomic orbitals to explain the...Ch. 9 - What hybridization is required for central atoms...Ch. 9 - Describe the bonding in H2S, CH4, H2CO and HCN...Ch. 9 - What hybridization is required for central atoms...Ch. 9 - Electrons in bonding molecular orbitals are most...Ch. 9 - What are molecular orbitals? How do they compare...Ch. 9 - Explain the difference between the and MOs for...Ch. 9 - Compare Figs. 4-47 and 4-49. Why are they...Ch. 9 - Which of the following would you expect to be more...Ch. 9 - Draw the Lewis structure for HCN. Indicate the...
Ch. 9 - Which is the more correct statement: The methane...Ch. 9 - Compare and contrast the MO model with the local...Ch. 9 - What are the relationships among bond order, bond...Ch. 9 - The molecules N2 and CO are isoelectronic but...Ch. 9 - Do lone pairs about a central atom affect the...Ch. 9 - In the hybrid orbital model, compare and contrast ...Ch. 9 - In the molecular orbital mode l, compare and...Ch. 9 - Why are d orbitals sometimes used to form hybrid...Ch. 9 - The atoms in a single bond can rotate about the...Ch. 9 - As compared with CO and O2, CS and S2 are very...Ch. 9 - Compare and contrast bonding molecular orbitals...Ch. 9 - What modification to the molecular orbital model...Ch. 9 - Why does the molecular orbital model do a better...Ch. 9 - The three NO bonds in NO3 are all equivalent in...Ch. 9 - Use the localized electron model to describe the...Ch. 9 - Use the localized electron model to describe the...Ch. 9 - Use the localized electron model to describe the...Ch. 9 - Use the localized electron model to describe the...Ch. 9 - The space-filling models of ethane and ethanol are...Ch. 9 - The space-filling models of hydrogen cyanide and...Ch. 9 - Give the expected hybridization of the central...Ch. 9 - Give the expected hybridization of the central...Ch. 9 - Give the expected hybridization of the central...Ch. 9 - Give the expected hybridization of the central...Ch. 9 - For each of the following molecules, write the...Ch. 9 - For each of the following molecules or ions that...Ch. 9 - Prob. 35ECh. 9 - The allene molecule has the following Lewis...Ch. 9 - Indigo is the dye used in coloring blue jeans. The...Ch. 9 - Urea, a compound formed in the liver, is one of...Ch. 9 - Biacetyl and acetoin are added to margarine to...Ch. 9 - Many important compounds in the chemical industry...Ch. 9 - Two molecules used in the polymer industry are...Ch. 9 - Hot and spicy foods contain molecules that...Ch. 9 - One of the first drugs to be approved for use in...Ch. 9 - Minoxidil (C9H15N15O) is a compound produced by...Ch. 9 - Consider the following molecular orbitals formed...Ch. 9 - Sketch the molecular orbital and label its type (...Ch. 9 - Which of the following are predicted by the...Ch. 9 - Which of the following are predicted by the...Ch. 9 - Using the molecular orbital model, write electron...Ch. 9 - Consider the following electron configuration:...Ch. 9 - Using the molecular orbital model to describe the...Ch. 9 - A Lewis structure obeying the octet rule can be...Ch. 9 - Using the molecular orbital model, write electron...Ch. 9 - Using the molecular orbital model, write electron...Ch. 9 - In which of the following diatomic molecules would...Ch. 9 - In terms of the molecular orbital model, which...Ch. 9 - Show how two 2p atomic orbitals can combine to...Ch. 9 - Show how a hydrogen 1s atomic orbital and a...Ch. 9 - Use Figs. 4-54 and 4-55 to answer the following...Ch. 9 - Acetylene (C2H2) can be produced from the reaction...Ch. 9 - Describe the bonding in NO+, NO, and NO, using...Ch. 9 - Describe the bonding in the O3 molecule and the...Ch. 9 - Describe the bonding in the CO32 ion using the...Ch. 9 - Draw the Lewis structures, predict the molecular...Ch. 9 - The antibiotic thiarubin-A was discovered by...Ch. 9 - Two structures can be drawn for cyanuric acid: a....Ch. 9 - Give the expected hybridization for the molecular...Ch. 9 - Vitamin B6 is an organic compound whose deficiency...Ch. 9 - Aspartame is an artificial sweetener marketed...Ch. 9 - Prob. 73AECh. 9 - The three most stable oxides of carbon are carbon...Ch. 9 - Complete the following resonance structures for...Ch. 9 - Prob. 77AECh. 9 - The transport of O2 in the blood is carried out by...Ch. 9 - Using molecular orbital theory, explain why the...Ch. 9 - Describe the bonding in the first excited state of...Ch. 9 - Using an MO energy-level diagram, would you expect...Ch. 9 - Show how a dxz. atomic orbital and a pz, atomic...Ch. 9 - What type of molecular orbital would result from...Ch. 9 - Consider three molecules: A, B, and C. Molecule A...Ch. 9 - Draw the Lewis structures for TeCl4, ICl5, PCl5,...Ch. 9 - A variety of chlorine oxide fluorides and related...Ch. 9 - Pelargondin is the molecule responsible for the...Ch. 9 - Complete a Lewis structure for the compound shown...Ch. 9 - Which of the following statements concerning SO2...Ch. 9 - Consider the molecular orbital electron...Ch. 9 - Place the species B2+ , B2, and B2 in order of...Ch. 9 - Consider the following computer-generated model of...Ch. 9 - Cholesterol (C27liu;O) has the following...Ch. 9 - Cyanamide (H2NCN), an important industrial...Ch. 9 - A flask containing gaseous N2 is irradiated with...Ch. 9 - Values of measured bond energies may vary greatly...Ch. 9 - Use the MO model to explain the bonding in BeH2....Ch. 9 - Prob. 101CPCh. 9 - Arrange the following from lowest to highest...Ch. 9 - Use the MO model to determine which of the...Ch. 9 - Given that the ionization energy of F2 is 290...Ch. 9 - Carbon monoxide (CO) forms bonds to a variety of...Ch. 9 - Prob. 106CPCh. 9 - As the bead engineer of your starship in charge of...Ch. 9 - Determine the molecular structure and...Ch. 9 - Although nitrogen trifluoride (NF3) is a thermally...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the hybridization of each central atom in the following molecules. (a) cyclohexene (b) phosgene, Cl2CO (c) glycine, H2NC(1)H2C(2)OOH (Note: Numbers in parentheses label each carbon atom.)arrow_forwardCalcium cyanamide, CaNCN, is used both to kill weeds and as a fertilizer. Give the Lewis structure of the NCN2 ion and the bonded-atom lone-pair arrangement and hybridization of the carbon atom.arrow_forwardThe structure of amphetamine, a stimulant, is shown below. (Replacing one H atom on the NH2, or amino, group with CH3 gives methamphetamine a particularly dangerous drug commonly known as speed.) (a) What are the hybrid orbitals used by the C atoms of the C6 ring. by the C atoms of the side chain, and by the N atom? (b) Give approximate values for the bond angles A, B, and C. (c) How many bonds and bonds are in the molerule? (d) Is the molecule polar or nonpolar? (e) Amphetamine reacts readily with a proton (H+) in aqueous solution. Where does this proton attach to the molecule? Explain how the electrostatic potential map predicts this site of protonation.arrow_forward
- Gamma hydroxybutyric acid, GHB, infamous as a date rape drug, is used illicitly because of its effects on the nervous system. The condensed molecular formula for GHB is HO(CH2)3COOH. (a) Write the Lewis structure for GHB. (b) Identify the hybridization of the carbon atom in the CH2 groups and of the terminal carbon. (c) Is hydrogen bonding possible in GHB? If so, write Lewis structures to illustrate the hydrogen bonding. (d) Which carbon atoms are involved in sigma bonds? In pi bonds? (e) Which oxygen atom is involved in sigma bonds? In pi bonds?arrow_forwardDo lone pairs about a central atom affect the hybridization of the central atom? If so, how?arrow_forwardWhy is the concept of hybridization required in valence bond theory?arrow_forward
- Minoxidil (C9H15N15O) is a compound produced by the Pharmacia Upjohn Company that has been approved as a treatment for some types of male pattern baldness. Note that in such shorthand ring structures, each point where lines meet is a carbon atom and that the hydrogen atoms bonded to the carbon atoms in the rings have been omitted. There will be four bonds to each carbon atom. a. Give the hybridization of the five nitrogen atoms in minoxidil. b. Give the hybridization of each of the nine carbon atoms in minoxidil. c. Give the approximate values for the bond angles marked a, b, c, d, e, and f. d. Including all the hydrogen atoms, how many bonds exist in minoxidil? e. How many bonds exist in minoxidil?arrow_forwardThe compound sketched below is acetylsalicylic acid, commonly known as aspirin. (a) What are the approximate values of the angles marked A, B, C, and D? (b) What hybrid orbitals are used by carbon atoms 1, 2, and 3ss?arrow_forwardThe sulfamate ion, H2NSO3, can be thought of as having been formed from the amide ion, NH2, and sulphur trioxide, SO3. (a) What are the electron-pair and molecular geometries or the amide ion and or SO3? What are the hybridizations of the N and S atoms, respectively? (b) Sketch a structure for the sulfamate ion, and estimate the bond angles. (c) What changes in hybridization do you expect for N and S in the course of the reaction NH2 + SO3 H2NSO3? (d) Is SO3 the donor of an electron pair or the acceptor of an electron pair in the reaction with amide ion? Does the electrostatic potential map shown below confirm your prediction?arrow_forward
- Methylcyanoacrylate is the active ingredient in super glues. Its Lewis structure is (a) How many sigma bonds are in the molecule? (b) How many pi bonds are in the molecule? (c) What is the hybridization of the carbon atom bonded to nitrogen? (d) What is the hybridization of the carbon atom bonded to oxygen? (e) What is the hybridization of the double-bonded oxygen?arrow_forwardConsider the polyatomic ion IO65-. How many pairs of electrons are around the central iodine atom? What is its hybridization? Describe the geometry of the ion.arrow_forwardSpecify the electron-pair and molecular geometry for each underlined atom in the following list. Describe the hybrid orbital set used by this atom in each molecule or ion. (a) CSe2 (b) SO2 (c) CH2O (d) NH4ssarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY