
Concept explainers
Vitamin B6 is an organic compound whose deficiency in the human body can cause apathy, irritability, and an increased susceptibility to infections. Below is an incomplete Lewis structure for vitamin B6. Complete the Lewis structure and answer the following questions. Hint: Vitamin B6 can be classified as an organic compound (a compound based on carbon atoms). The majority of Lewis structures for simple organic compounds have all atoms with a formal charge of zero. Therefore, add lone pairs and multiple bonds to the structure below to give each atom a formal charge of zero.
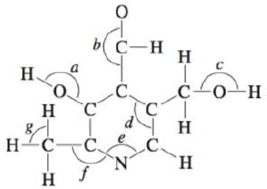
a. How many σ bonds and π bonds exist in vitamin B6?
b. Give approximate values for the bond angles marked a through g in the structure.
c. How many carbon atoms are sp2 hybridized?
d. How many carbon, oxygen, and nitrogen atoms are sp3 hybridized?
e. Does vitamin B6 exhibit delocalized π bonding? Explain.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Chemistry
- Write Lewis structures for these ions. Show all valence electrons and all formal charges. (a) Amide ion, NH2 (b) Bicarbonate ion, HCO3 (c) Carbonate ion, CO32 (d) Nitrate ion, NO3 (e) Formate ion, HCOO (f) Acetate ion, CH3COOarrow_forwardThe study of carbon-containing compounds and their properties is called organic chemistry. Besides carbon atoms, organic compounds also can contain hydrogen, oxygen, and nitrogen atoms (as well as other types of atoms). A common trait of simple organic compounds is to have Lewis structures where all atoms have a formal charge of zero. Consider the following incomplete Lewis structure for an organic compound called histidine (an amino acid), which is one of the building blocks of proteins found in our bodies: Draw a complete Lewis structure for histidine in which all atoms have a formal charge of zero.arrow_forwardAspartame is an artificial sweetener marketed under the name Nutra-Sweet. A partial Lewis structure for aspartame is shown below. Aspartame can be classified as an organic compound (a compound based on carbon atoms). The majority of Lewis structures for simple organic compounds have all atoms with a formal charge of zero. Therefore, add lone pairs and multiple bonds to the structure above to give each atom a formal charge of zero when drawing the Lewis structure. Also note that the six-sided ring is shorthand notation for a benzene ring (C6H5). Benzene is discussed in Section 4-7. Complete the Lewis structure for aspartame. How many C and N atoms exhibit sp1 hybridization? How many C and O atoms exhibit sp3 hybridization? How many and bonds are in aspartame?arrow_forward
- A complete Lewis structure must show all nonzero formal charges. Complete each of thefollowing Lewis structures by adding any missing formal charges.arrow_forwardUnshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. The number of unshared pairs at atom a is The number of unshared pairs at atom b is H3C The number of unshared pairs at atom c is The number of unshared pairs at atom a is H c a H2N-C+ The number ofunshared pairs at atom b is CH3 The number of unshared pairs at atom c isarrow_forwardHow do you know when to draw a solid wedge vs a dashed wedge when drawing 3D bond-line structures? I know that solid-wedge means the atom is pointing towards you and dashed wedge means it's in the back, but how do you know which atoms are in the front as opposed to the back? How can you tell what the configuration will look like in space just by looking at the lewis structure or name?arrow_forward
- Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. The number of unshared pairs at atom a is CH3 The number of unshared pairs at atom b is The number of unshared pairs at atom c is The number of unshared pairs at atom a is v. The number of unshared pairs at atom b is The number of unshared pairs at atom c is HC CNH₂ a Nb Varrow_forwardA resonance hybrid is a structure that can be depicted by more than one valid Lewis structure. part1: Draw the major resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons. part2: Draw the second most important resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons. part3: Draw the least important resonance contributor for fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized and should include all nonzero formal charges and all nonbonding electrons.arrow_forwardA resonance hybrid is a structure that can be depicted by more than one valid Lewis structure. part1: Draw the major resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons. part2: Draw the second most important resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons.arrow_forward
- Draw a Lewis structure for the compound whose skeletal structure is provided to you below. Don't forget to draw in the H atoms! CH₂ HC CH 11 HC — CH, - Draw the Lewis structure by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons.arrow_forwardUnshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. 201 H₂C The number of unshared pairs at atom a is 3. The number of unshared pairs at atom b is ov The number of unshared pairs at atom c is 1 The number of unshared pairs at atom a is [2 The number of unshared pairs at atom b is ov The number of unshared pairs at atom c is 2arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure :C=0: :Z: :Z: N=N #] C=N Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: * * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forward

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





