
Concept explainers
Interpretation:
The balanced reaction between NO and O2 and the limiting reactant needs to be predicted.
Concept Introduction:
The reaction in which the reactant is totally consumed when the reaction is completed is known as limiting reactant
Answer to Problem 49A
The balanced reaction is shown below:
NO will act as a limiting reactant.
Explanation of Solution
In the given reaction is shown below:
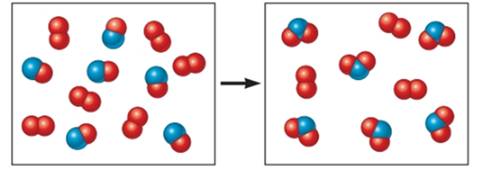
The O2 molecules represented as 
The NO molecules represented as 
The NO2 molecules represented as 
In the reactant side there is O2 and NO molecules present in the reaction whereas in the product side there are NO2 molecules present.
The reaction is shown below:
The balance
In the given reaction; 6 molecules of O2 reacts with 6 molecules of NO in the reactant side.
According to the given reaction 1 molecule of O2 reacts with 2 molecules of NO
Therefore, 6 molecules of O2 would react with 12 molecules of NO. But there are 6 molecules of NO that means NO will get totally consumed in the reaction and thus, will act as a limiting reactant.
Thus, the balanced reaction is shown below:
NO will act as a limiting reactant.
Chapter 9 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





