
Concept explainers
Thinking Mechanistically About
The preparation and properties of alkynes extend some topics explored in earlier chapters:
* Alkynes can be prepared by elimination reactions related to the
* Alkynes can be prepared by
* Alkynes undergo addition reactions, especially electrophilic addition, with many of the same compounds that add to alkenes.
The greater s character of sp hybrid orbitals compared with
* The
* Unlike alkenes, alkynes are reduced by metals, especially
* Unlike alkenes, alkynes can undergo nucleophilic as well as electrophilic addition.

Problems
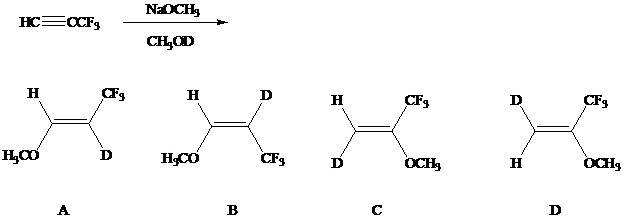
Nucleophilic addition can occur with alkynes that bear strong electron-attractingsubstituents such as
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Organic Chemistry - Standalone book
- Which of the following reagents would be used as a chemical test for the presence of alkenes and alkynes? O a. Br2 O b. H2O О с. HCl O d. CH3OHarrow_forward3. Please draw the most important (stable) resonance form of the carbonation that mediates the following reaction: OH H3O+ O 4. Draw the structure of the alkene isomer (with the molecular formula of C5H10) that is most reactive one in the addition reaction with Br₂.arrow_forward10. The relative rates of epoxidation reactions of the following four alkenes are 1, 22, 484, 6526. Which alkene does feature the relative epoxidation rate of 484? 11. Alkynes undergo addition reaction with HCl, forming corresponding alkenyl bromide. Please write the rate law of such a reaction between butyne and HCl.arrow_forward
- 10) Synthesis: Make the following products from a suitable cyclic alkene starting material. Look at the functional group PATTERN present in the molecule, including stereochemistry. ♡ Br Brarrow_forwardAlkyl sulfonates undergo the same type of substitution reactions as alkyl halides and can also be prepared from alcohols. What advantage does the preparation of an alkyl sulfonate from an alcohol have over the preparation of an alkyl halide from an alcohol?arrow_forward5B In the following reactions, mixtures of alkenes and ethyl ethers are formed. Draw their structures. Explain which is or are likely to be the main product(s) in each reaction. In case of formation of two isomers of alkenes, explain which is formed in greater proportion CH3 CH3 H3C-C H -Br CH3 EtOHarrow_forward
- 2-chloropropane is a major product of the reaction of chlorine with propane under ultraviolet light. Write the mechanism for this reaction including the initiation step and the two propagation steps.arrow_forward5A In the following reactions, mixtures of alkenes and ethyl ethers are formed. Draw their structures. Explain which is or are likely to be the main product(s) in each reaction. In case of formation of two isomers of alkenes, explain which is formed in greater proportion CH3 ofi H3C- -Br CH3 EtOHarrow_forwardIt contains a vinyl group attached to benzene. Imagine it reacting with KMNO4 in acidic medium. What is/are the product(s)? A 1-phenylethane-1,2-diol B 2 moles of carbon dioxide C Carbon dioxide and benzoic acid D Carbon dioxide and benzaldehydearrow_forward
- Write structural formulas for all the alkenes that can be formed in the reaction of 2-bromobutane with potassium ethoxide (KOCH2CH3).arrow_forward1. Reaction of 2,3-dimethyl-1-butene with HBr leads to an alkyl bromide, C6H13Br. On treatment of this alkyl halide with KOH in methanol, elimination of HBr occurs and a hydrocarbon that is isometric with the starting alkene is formed. What is the structure of this hydrocarbon and how do you think it is formed from an alkyl bromide? 2. 1-Octen-3-ol is a potent mosquito attractant commonly used in mosquito traps. A number of reactions, including hydrogenation, will transform 1-Octen-3-ol into a less effective molecule. Write a complete reaction equation for the hydrogenation of this alkenol.arrow_forwardThe reaction of Hbr with 2-methylpropene produces 2-bromo-2-methylpropane. What is the structure of the carbocation formed during the reaction?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
