
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 3PP
PRACTICE PROBLEM 9.3
Using the method of Section 9.5A, determine the number of expected signals for the following compounds.
(a)
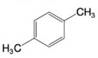
(b)
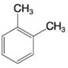
(c)
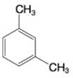
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show work. don't give Ai generated solution
None
None
Chapter 9 Solutions
Organic Chemistry
Ch. 9 - Prob. 1PPCh. 9 - PRACTICE PROBLEM 9.2 What compound with molecular...Ch. 9 - PRACTICE PROBLEM 9.3
Using the method of Section...Ch. 9 - PRACTICE PROBLEM 9.4 How many signals would each...Ch. 9 - Prob. 5PPCh. 9 - Prob. 6PPCh. 9 - PRACTICE PROBLEM 9.7
The relative chemical shifts...Ch. 9 - Prob. 8PPCh. 9 - PRACTICE PROBLEM 9.9 Propose a structure for...Ch. 9 - PRACTICE PROBLEM 9.10
What is the dihedral angle...
Ch. 9 - PRACTICE PROBLEM 9.11 Draw the most stable chair...Ch. 9 - Prob. 12PPCh. 9 - PRACTICE PROBLEM 9.13 How many signals would you...Ch. 9 - Prob. 14PPCh. 9 - Prob. 15PPCh. 9 - Prob. 16PPCh. 9 - Prob. 17PPCh. 9 - PRACTICE PROBLEM 9.18
What are the expected ratios...Ch. 9 - Prob. 19PPCh. 9 - How many 1H NMR signals (not peaks) would you...Ch. 9 - How many 13C NMR signals would you predict for...Ch. 9 - Prob. 22PCh. 9 - Prob. 23PCh. 9 - Prob. 24PCh. 9 - Compound Q has the molecular formula C7H8. The...Ch. 9 - 9.26 Explain in detail how you would distinguish...Ch. 9 - Compound S (C8H16) reacts with one mole of bromine...Ch. 9 - A compound with molecular formula C4H8O has a...Ch. 9 - In the mass spectrum of 2, 6-dimethyl-4-heptanol...Ch. 9 - Prob. 30PCh. 9 - What are the masses and structures of the ions...Ch. 9 - Prob. 32PCh. 9 - Ethyl bromide and methoxybenzene (shown below)...Ch. 9 - 9.34 The homologous series of primary amines, ,...Ch. 9 - Propose a structure that is consistent with each...Ch. 9 - 9.36 Propose structures for compounds E and F....Ch. 9 - 9.37 Use the NMR and IR data below to propose a...Ch. 9 - 9.38 When dissolved in , a compound (K) with the...Ch. 9 - Compound T (C5H8O) has a strong IR absorption band...Ch. 9 - Prob. 40PCh. 9 - Deduce the structure of the compound that gives...Ch. 9 - Deduce the structure of the compound that gives...Ch. 9 - The 1H NMR spectrum of a solution of 1,...Ch. 9 - Acetic acid has a mass spectrum showing a...Ch. 9 - The 1H NMR peak for the hydroxyl proton of...Ch. 9 - The 1H NMR study of DMF (N, N-dimethylformamide)...Ch. 9 - 9.48 The mass spectra of many benzene derivatives...Ch. 9 - Prob. 49PCh. 9 - 1. Given the following information, elucidate the...Ch. 9 - Two compounds with the molecular formula C5H10O...
Additional Science Textbook Solutions
Find more solutions based on key concepts
4. What five specific threats to biodiversity are described in this chapter? Provide an example of each.
Biology: Life on Earth (11th Edition)
24.19 (a) On the basis of the following sequence of reactions, Emil Fischer was able to show that -serine and -...
Organic Chemistry
8. A classic way to isolate thymidylate synthase—negative mutants of bacteria is to treat a growing culture wit...
Biochemistry: Concepts and Connections (2nd Edition)
An inventor has built an engine X and claims that its efficiency X is greater than the efficiency of an ideal ...
Fundamentals of Physics Extended
Describe the 1H NMR spectrum you would expect for each of the following compounds, indicating the relative posi...
Organic Chemistry (8th Edition)
The bioremediation process shown in the photograph is used to remove benzene and other hydrocarbons from soil c...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the correct common name for the compound shown here.arrow_forwardPh heat heatarrow_forward(12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY