
Concept explainers
For each of the following molecules or ions that contain sulfur, write the Lewis structure(s), predict the molecular structure (including bond angles), and give the expected hybrid orbitals for sulfur.
a. SO2
b. SO3
c. 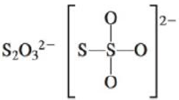
d. 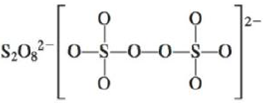
e. SO32−
f. SO42−
g. SF2
h. SF4
i. SF6
j. F3S—SF
k. SF5+
(a)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on each sulfur and oxygen atom. Two oxygen atoms are bonded to sulfur atom. Therefore, the total valence electrons are
Therefore the geometry is bent. The bond angle is less than
The Lewis structure of
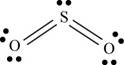
Figure 1
(b)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Two oxygen atoms are attached to sulfur, therefore, the total number of valence electrons is
The molecule has trigonal planar geometry with bond angle
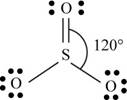
Figure 2
(c)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Three oxygen atoms and one sulfur atom is attached to central sulfur atom and charge on the molecule is
By bonding in this way, they complete their octet. The molecular structure is tetrahedral with bond angle approximately equal to
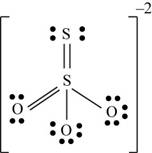
Figure 3
(d)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. There are eight oxygen atoms and two sulfur atoms are present in the molecule and charge on the molecule is
The two oxygen atoms in the centre are bonded by single bond. By bonding in this way, they complete their octet. The molecular structure is tetrahedral with bond angle approximately equal to
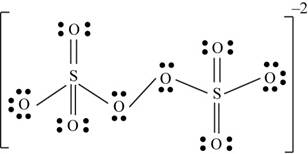
Figure 4
(e)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Three oxygen atoms and one sulfur atom present in the molecule and charge on the molecule is
One oxygen atom is single bonded with sulfur and one is joined by pi bond. By bonding in this way, they complete their octet. The molecular structure is trigonal pyramidal with bond angle approximately equal to
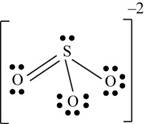
Figure 5
(f)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Four oxygen atoms and one sulfur atom is present in the molecule and charge on the molecule is
Two oxygen atoms are single bonded with sulfur and two joined by pi bond. By bonding in this way, they complete their octet. The molecular structure is tetrahedral with bond angle approximately equal to
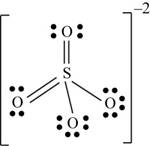
Figure 6
(g)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Two fluorine atoms and one sulfur atom is present in the molecule, therefore, the total number of valence electrons is
The sulfur is bonded to two fluorine atoms by sigma bond. By bonding in this way, they complete their octet. The molecular structure is bent due to presence of lone pairs of electrons on sulfur. The bond angle is less than
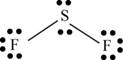
Figure 7
(h)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Four fluorine atoms and one sulfur atom is present in the molecule, therefore, the total number of valence electrons is
The molecular structure is see-saw due to presence of lone pair of electrons on sulfur. The equatorial bond angles are
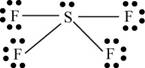
Figure 8
(i)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Six fluorine atoms and one sulfur atom is present in the molecule, therefore, the total number of valence electrons is
The molecular structure is octahedral with bond angle
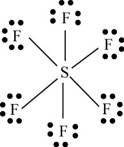
Figure 9
(j)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Four fluorine atoms and two sulfur atoms are present in the molecule, therefore, the total number of valence electrons is
The molecular structure is see-saw due to presence of lone pair of electrons on sulfur. The equatorial bond angles are
The Lewis structure of
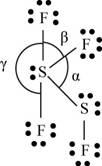
Figure 10
(k)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Five fluorine atoms and one sulfur atom is present in the molecule and charge on the molecule is
The molecular structure is trigonal bipyramidal with equatorial bond angles
The Lewis structure of
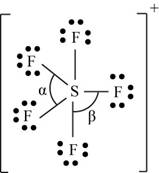
Figure 11
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry
- hy do atoms form bonds with one another? What can make a molecule favored compared with the lone atoms?arrow_forwardLets look more closely at the process of hybridization. (a) What is the relationship between the number of hybrid orbitals produced and the number of atomic orbitals used to create them? (b) Do hybrid atomic orbitals form between different p orbitals without involving 5 orbitals? (c) What is the relationship between the energy of hybrid atomic orbitals and the atomic orbitals from which they are formed?arrow_forwardFor each of the following molecular compounds, identify the atom that is most likely to be found in the center of its Lewis structure. a. CBr4b. SO2c. H2S d. NOF e. BI3f. SO3g. AsH3h. HCNarrow_forward
- What is the hybridization state of the Boron in BCl2Br? And is this a polar or nonpolar molecule? a. sp and polar b. sp3 and nonpolar c. sp2 and polar d. sp3 and polar e. sp2 and nonpolararrow_forward3. Draw all the possible resonance structures for each of the following molecules. a. SO₂ b. NO3 4. Draw Lewis structures for the following molecules. Describe how the bond angles would be altered based on the Lewis structure. a. CH₂S b. SC1₂ 9arrow_forwardWhat are valid uses of dummy atoms? A. They are needed to define dihedral angles when three or more bonded atoms are in a straight line B. They are needed to define bond angles in linear distributions of atoms in a molecule C. They can help build highly symmetrical structures D. They are used when a molecule has very heavy atoms O a. A, D b. C, D О с. A, C O d. B, Darrow_forward
- Given the Lewis structures below for the three molecules labeled A, B, and C, what is the molecular geometry around the central atom in each? (NOTE: Lewis structure drawings may not accurately represent the molecular geometry) A. B. H-As-H Se 第一 H. Molecule A V [Choose ] see-saw octahedral Molecule B linear bent trigonal planar Molecule C square pyramidal T-shaped square planar tetrahedral trigonal pyramidalarrow_forwardWhich molecule or ion does not belong? (has a shape different from the rest of them)? a. NF3 b. CH2O c. C2H4 d. SO3arrow_forwardWhich of the following molecules will have a Lewis dot structure with exactly one unshared electron pair on the central atom? a. H₂O b. PH3 c. PCl5 d. CH₂Cl₂ e. BeCl₂arrow_forward
- Write a Lewis structure for each of the following simple molecules. Show all bonding valenceelectron pairs as lines and all nonbonding valence electron pairs as dots.a. H2 b. HCl c. CF4 d. O2arrow_forwardConsider the molecules PF3 and PF5. A. Draw the Lewis electron-dot structures for PF3 and PF5 and predict the molecular geometry. B. Is the PF3 polar or non-polar and why? C. On the basis of bonding principles, predict whether each of the following compounds exists. In each case, explain your prediction. (i) NF5 (ii) AsF5arrow_forwardThe skeleton structure of atomic chain in 2-aminoacetaldehyde (NH2CH2CHO) is N–C–C–O . The complete Lewis structure of this compound has A. 3 lone pairs, B. 1 π bond and 8 σ bonds. C. 1 lone pair, 1 π bond and 7 σ bonds.D. 3 lone pairs, 1 π bond and 7 σ bonds. E. 1 lone pair, 1 π bond and 8 σ bonds. F 2 lone pairs, 1 π bond and 8 σ bonds.arrow_forward

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





