
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 24E
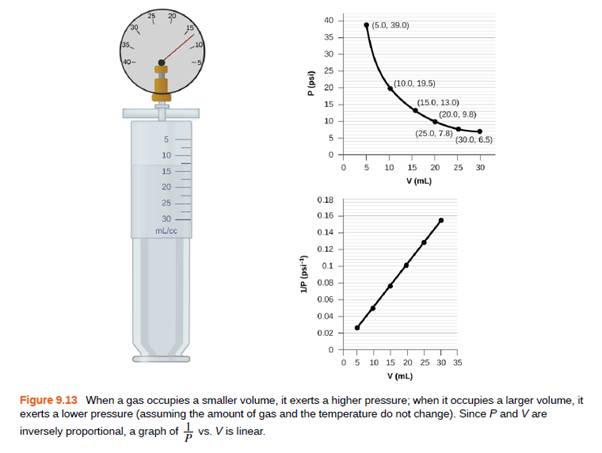
In addition to the data found in Figure 9.13, what other information do we need to find the mass of the sample of air used to determine the graph?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
(12) Which one of the following statements about fluo-
rometry is FALSE?
a) Fluorescence is better detected at 90 from the exci-
tation direction.
b) Fluorescence is typically shifted to longer wave-
length from the excitation wavelength.
c) For most fluorescent compounds, radiation is pro-
duced by a
transition
Don't used Ai solution
Don't used Ai solution
Chapter 9 Solutions
Chemistry by OpenStax (2015-05-04)
Ch. 9 - Why are sharp knives more effective than dull...Ch. 9 - Why do some small bridges have weight limits that...Ch. 9 - Why should you roll or belly-crawl rather than...Ch. 9 - A typical barometric pressure in Redding....Ch. 9 - A typical barometric pressure in Denver, Colorado,...Ch. 9 - A typical barometric pressure in Kansas City is...Ch. 9 - Canadian tire pressure gauges are marked in units...Ch. 9 - Dining the Viking landings on Mars, the...Ch. 9 - The pressure of the atmosphere on the surface of...Ch. 9 - A medical laboratory catalog describes the...
Ch. 9 - Consider this scenario and answer the following...Ch. 9 - Why is it necessary to use a nonvolatile liquid in...Ch. 9 - The pressure of a sample of gas is measured at sea...Ch. 9 - The pressure of a sample of gas is measured with...Ch. 9 - The pressure of a sample of gas is measured at sea...Ch. 9 - The pressure of a sample of gas ¡s measured a sea...Ch. 9 - How would the use of a volatile liquid affect the...Ch. 9 - Sometimes leaving a bicycle in the sun on a hot...Ch. 9 - Explain how the volume of the bubbles exhausted by...Ch. 9 - One way to state Boyle’s law is All other things...Ch. 9 - An alternate way to state Avogadro’s law is A1l...Ch. 9 - How would the graph in Figure 9.12 change if the...Ch. 9 - How would the graph in Figure 9.13 change if the...Ch. 9 - In addition to the data found in Figure 9.13, what...Ch. 9 - Determine the volume of 1 mol of CH4 gas at 150 K...Ch. 9 - Determine the pressure of the gas in the syringe...Ch. 9 - A spray can is used until it is empty except for...Ch. 9 - What is the temperature of an 11.2-L sample of...Ch. 9 - À 2.50-L volume of hydrogen measured at —196 C is...Ch. 9 - A balloon inflated with three breaths of air has a...Ch. 9 - A weather balloon contains 8.80 moles of helium at...Ch. 9 - The volume of an automobile air bag was 66.8 L...Ch. 9 - How many moles of gaseous boron trifluoride, BF3,...Ch. 9 - Iodine, I2, is a solid at room temperature but...Ch. 9 - How many grams of gas are present in each of the...Ch. 9 - A high altitude balloon is filled with 1041104 L...Ch. 9 - A cylinder of medical oxygen has a volume of 3S.4...Ch. 9 - A large scuba tank (Figure 9.16) with a volume of...Ch. 9 - A 20.0-L cylinder containing 11.34 kg of butane,...Ch. 9 - While resting, the average 70-kg human male...Ch. 9 - For a given amount of gas showing ideal behavior,...Ch. 9 - A liter of methane gas, CH4, at STP contains more...Ch. 9 - The effect of chlorofluorocarbons (such as CCl2F2)...Ch. 9 - As 1 g of (lie radioactive element radium decays...Ch. 9 - A balloon that is 100.21 L at 21 C and 0.981 atm...Ch. 9 - If the temperature of a fixed amount of a gas is...Ch. 9 - If the volume of a fixed amount of a gas is...Ch. 9 - What is the density of laughing gas, dinitrogen...Ch. 9 - Calculate the density of Freon 12, CF2Cl2, at 30.0...Ch. 9 - Which is denser at the same temperature and...Ch. 9 - A cylinder of O2(g) used in breathing by emphysema...Ch. 9 - What is the molar mass of a gas if 0.0494 g of the...Ch. 9 - What is the molar mass of a gas if 0.281 g of the...Ch. 9 - How could you show experimentally that the...Ch. 9 - The density of a certain gaseous fluoride of...Ch. 9 - Consider this question: What is the molecular...Ch. 9 - A 36.0—L cylinder of a gas used for calibration of...Ch. 9 - A cylinder of a gas mixture used for calibration...Ch. 9 - A sample of gas isolated from unrefined petroleum...Ch. 9 - A mixture of 0.200 g of 1.00 g of and 0.820 g of...Ch. 9 - Most mixtures of hydrogen gas with oxygen gas are...Ch. 9 - A commercial mercury vapor analyzer can detect in...Ch. 9 - A sample of carbon monoxide was collected over...Ch. 9 - In an experiment in a general chemistry...Ch. 9 - Joseph Priestley first prepared pure oxygen by...Ch. 9 - Cavendish prepared hydrogen in 176G by the novel...Ch. 9 - The chlorofluorocarbon CCl2F2 can be recycled into...Ch. 9 - Automobile air bags are inflated with nitrogen...Ch. 9 - Lime, CaO, is produced by heating calcium...Ch. 9 - Before small batteries were available, carbide...Ch. 9 - Calculate the volume of oxygen required to burn...Ch. 9 - What volume of O2 at STP is required to oxidize...Ch. 9 - Consider the following questions: (a) What is the...Ch. 9 - Methanol, CH3OH, is produced industrially by the...Ch. 9 - What volume of oxygen a 423.0 K and a pressure of...Ch. 9 - A 230-L sample of a colorless gas at STP...Ch. 9 - Ethanol, C2H5OH, is produced industrially from...Ch. 9 - One molecule of hemoglobin will combine with four...Ch. 9 - A sample of a compound of xenon and fluorine was...Ch. 9 - One method of analyzing amino acids is the van...Ch. 9 - A balloon filled with helium gas is found to take...Ch. 9 - Explain why the numbers of molecules are not...Ch. 9 - Starting with the definition of rate of effusion...Ch. 9 - Heavy water, D2O (molar mass = 20.03 g mol-1). can...Ch. 9 - Which of the following gases diffuse more slowly...Ch. 9 - During the discussion of gaseous diffusion for...Ch. 9 - Calculate the relative rate of diffusion of 1H2...Ch. 9 - A gas of unknown identity diffuses at a rate of...Ch. 9 - When two cotton plugs. one moistened with ammonia...Ch. 9 - Using the postulates of the kinetic molecular...Ch. 9 - Can the speed of a given molecule in a gas double...Ch. 9 - Describe what happens o the average kinetic energy...Ch. 9 - The distribution of molecular velocities in a...Ch. 9 - What is the ratio of the average kinetic energy of...Ch. 9 - A 1-L sample of CO initially at STP is heated to...Ch. 9 - The root mean square speed of H2, molecules at 25...Ch. 9 - Answer the following questions: (a) Is the...Ch. 9 - Show that the ratio of the rate of diffusion of...Ch. 9 - Graphs showing the behavior of several different...Ch. 9 - Explain why the plot of PV for CO2 differs from...Ch. 9 - Under which of the following sets of conditions...Ch. 9 - Describe the factors responsible for the deviation...Ch. 9 - For which of the following gases should the...Ch. 9 - A 0.245-L flask contains 0.467 mol CO2 at 159 C....Ch. 9 - Answer the following questions: (a) If XX behaved...
Additional Science Textbook Solutions
Find more solutions based on key concepts
21. The accompanying pedigree shows the transmission of a phenotypic character. Using B to represent a dominan...
Genetic Analysis: An Integrated Approach (3rd Edition)
An atom with a formal charge does not necessarily have more or less electron density than the atoms in the mole...
Organic Chemistry (8th Edition)
Use a globe or map to determine, as accurately as possible, the latitude and longitude of Athens, Greece.
Applications and Investigations in Earth Science (9th Edition)
WHAT IF? As a cell begins the process of dividing, its chromosomes become shorter, thicker, and individually vi...
Campbell Biology in Focus (2nd Edition)
17. Anthropologists are interested in locating areas in Africa where fossils 4-8 million years old might be fou...
Campbell Biology: Concepts & Connections (9th Edition)
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used Ai solutionarrow_forwardIndicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
- 1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER