
Concept explainers
(a)
Interpretation:
The product formed from the reaction has to be given and the limiting agent has to be given.
The reaction is,
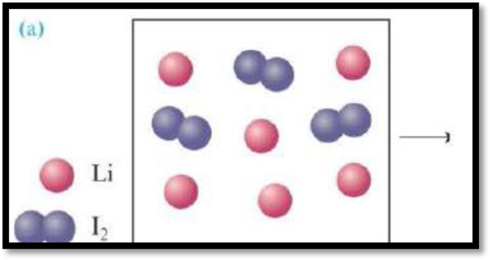
Figure 1
Concept Introduction:
The limiting reactant of the reaction is the reactant that is completely used during the reaction. Using the mole ratio and starting amounts of the reactants limiting reactant can be determined.
Example:
Consider a reaction starts with
(b)
Interpretation:
The product formed from the reaction has to be given and also the limiting agent has to be given.
The reaction is,
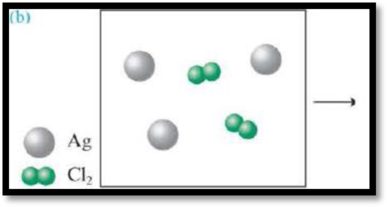
Figure 3
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- For the chemical reaction C3H8O2+4O23CO2+4H2O how many product molecules are formed when nine C3H8O2 molecules react?arrow_forward4.8 In an experiment carried out at very low pressure, 13x1015 molecules of H2 are reacted with acetylene, C2H2, to form ethane, C2H6, on the surface of a catalyst. Write a balanced chemical equation for this reaction. How many molecules of acetylene are consumed?arrow_forwardCalculate the amounts of reactants needed in a chemical reaction to produce a specified amount of product.arrow_forward
- For this reaction, fill in the table with the indicated quantities for the balanced equation. 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)arrow_forwardConsider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. a Write a balanced chemical equation representing this reaction. b Write a word description of the reaction on the particulate and molar levels.arrow_forwardlist at least three quantities that must be conserved in chemical reactions.arrow_forward
- 4.37 The theoretical yield and the actual yield for various reactions are given below. Determine the corresponding percentage yields. Theoretical Yield Actual Yield Reaction 1 35.0 g 12.8 g Reaction 2 9.3 g 120 mg Reaction 3 3.7 metric tons 1250 kg Reaction 4 40.0 g 41.0 garrow_forwardClassify each of the following statements as true or false: a Coefficients in a chemical equation express the molar proportions among both reactants and products. b A stoichiometry problem can be solved with an unbalanced equation. c In solving a stoichiometry problem, the change from quantity of given substance to quantity of wanted substance is based on masses. d Percentage yield is actual yield expressed as a percentage of ideal yield. e The quantity of product of any reaction can be calculated only through the moles of the limiting reactant. f rH is positive for an endothermic reaction and negative for an exothermic reaction.arrow_forwardCarbon dioxide from the atmosphere weathers, or dissolves, limestone (CaCO3) by the reaction CaCO3(s)+CO2(g)+H2O(l)Ca2(aq)+2HCO3(aq) Obtain H for this reaction. See Table 6.2 for the data.arrow_forward
- Write an equation from the following description: reactants are gaseous NH3 and O2, products are gaseous NO2 and liquid H2O, and the stoichiometric coefficients are 4, 7, 4, and 6, respectively.arrow_forward4.72 The picture shown depicts the species present at the start of a combustion reaction between methane, CH4 and oxygen, O2 (a) What is the limiting reactant? (b) Draw the resulting state after this set of reactants has reacted as far as possible.arrow_forwardThe carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide 2LiOH(s)+CO2(g)Li2CO3(s)+H2O(l) Estimate the grams of lithium hydroxide required per astronaut per day. Assume that each astronaut requires 2.50 103 kcal of energy per day. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. The H for glucose(s) is 1273 kJ/mol.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





