
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8.8, Problem 8.60P
Interpretation Introduction
Interpretation:
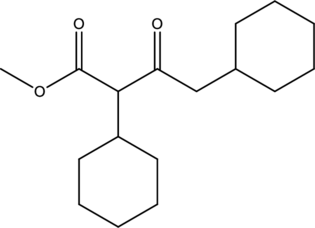
Reagents that are required to obtain the given compound using claisen condensation has to be identified.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The compound shown is the principal component of vanilla bean extracts:
H
HO
What is the IUPAC name of this compound?
O 1-formyl-3-methoxyphenol
O 3-formyl-2-methoxyphenol
O 4-hydroxy-3-methoxybenzaldehyde
O
1-hydroxy-2-methoxybenzaldehyde
O None of these
OCH 3
18-46
Procaine (its hydrochloride salt is marketed as Novocaine) was one of the first local anesthetics developed for infiltration and regional anesthesia. it is synthesized by the following fischer esterification:
What aldehyde or ketone would be obtained when the following compound is heated in a basic aqueous solution?
2,4-dicyclohexyl-3-hydroxybutanal
Chapter 8 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.2 - Prob. 8.9PCh. 8.2 - Prob. 8.10PCh. 8.2 - Prob. 8.11PCh. 8.2 - Prob. 8.12P
Ch. 8.2 - Prob. 8.13PCh. 8.3 - Predict the products of each of the following...Ch. 8.3 - Predict the products of each of the following...Ch. 8.3 - Predict the products of each of the following...Ch. 8.3 - Predict the products of each of the following...Ch. 8.4 - Prob. 8.20PCh. 8.4 - Prob. 8.21PCh. 8.4 - Prob. 8.22PCh. 8.4 - Prob. 8.23PCh. 8.5 - Prob. 8.25PCh. 8.5 - Prob. 8.26PCh. 8.5 - On a separate piece of paper, draw a mechanism for...Ch. 8.6 - Prob. 8.29PCh. 8.6 - Predict the major product for each of the...Ch. 8.6 - Predict the major product for each of the...Ch. 8.6 - Predict the major product for each of the...Ch. 8.6 - Identify the reagents you would use to achieve...Ch. 8.6 - Identify the reagents you would use to achieve...Ch. 8.6 - Identify the reagents you would use to achieve...Ch. 8.7 - Predict the major product for each of the...Ch. 8.7 - Predict the major product for each of the...Ch. 8.7 - Predict the major product for each of the...Ch. 8.7 - Prob. 8.42PCh. 8.7 - Prob. 8.43PCh. 8.7 - Prob. 8.44PCh. 8.7 - Prob. 8.45PCh. 8.7 - Prob. 8.47PCh. 8.7 - Prob. 8.48PCh. 8.7 - Prob. 8.49PCh. 8.7 - Prob. 8.50PCh. 8.8 - Prob. 8.52PCh. 8.8 - Prob. 8.53PCh. 8.8 - Prob. 8.54PCh. 8.8 - Prob. 8.55PCh. 8.8 - Prob. 8.57PCh. 8.8 - Prob. 8.58PCh. 8.8 - Prob. 8.59PCh. 8.8 - Prob. 8.60PCh. 8.8 - Propose a mechanism for each of the following...Ch. 8.8 - Propose a mechanism for each of the following...Ch. 8.8 - Prob. 8.64PCh. 8.9 - Prob. 8.66PCh. 8.9 - Prob. 8.67PCh. 8.9 - Prob. 8.68PCh. 8.9 - Prob. 8.69PCh. 8.9 - Prob. 8.70PCh. 8.9 - Prob. 8.71PCh. 8.9 - Prob. 8.72PCh. 8.9 - Identify what reagents you would use to achieve...Ch. 8.9 - Identify what reagents you would use to achieve...Ch. 8.9 - Identify what reagents you would use to achieve...Ch. 8.10 - Prob. 8.78PCh. 8.10 - Prob. 8.79PCh. 8.10 - Prob. 8.80PCh. 8.10 - Propose a synthesis for each of the following...Ch. 8.10 - Propose a synthesis for each of the following...Ch. 8.10 - Propose a synthesis for each of the following...Ch. 8.10 - Propose a synthesis for each of the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following will react with Tollen's reagent? 요 CH₂CH OH CH₂ C CH3 CH3 OCH3CH₂OCH₂CH3 CH₂-C-CH₂arrow_forwardWhat product is formed from the addition of NaOH to p-tertbutylphenol? Group of answer choices a compound with addition of an OH group to p-tertbutyl phenol the sodium salt of p-tertbutylphenol, sodium p-tertbutylphenoxide a protonated version of p-tertbutylphenol there is no reactionarrow_forwardIdentify the reagents you may use to do the following synthesis:arrow_forward
- Using your reaction roadmaps as a guide, show how to convert propane into propyl propanoate. You must use propane as the source of all carbon atoms in the target molecule. Show all reagents needed and all molecules synthesized along the way.arrow_forwardDraw the products obtained when each of the following ethers is heated with one equivalent of HBr:arrow_forwardWhat products are formed by acidic hydrolysis of the following compound? benzaldehyde and butan-1-d benzeie acid and butan-2-d 2-methylbutanoic acid and phenol benzoic acid and methyl ethyl ketonearrow_forward
- Identify the reagents you would use to prepare the following compound via a Robinson annulation: ⒸHCI, heat O O NaOH, heat H IIarrow_forwardWhat products will be obtained if the following compound is hydrolyzed completely in an aqueous solution of HCL?arrow_forwardWhich of the following sequences of reactions would convert toluene to 2-bromo-4-cyanotoluene? ? Br CN Nitration, bromination, reduction, diazotization, reaction with cyanide anion Bromination, nitration, reduction, diazotization, reaction with cyanide anion Bromination, nitration, diazotization, reduction , reaction with cyanide anion Nitration, bromination, diazotization, reduction, reaction with cyanide anionarrow_forward
- Which of the following compounds will not undergo keto-enol tautomerism? ОН OH ОН OH ОНarrow_forwardUsing cyclohexanone as the starting material, describe how the following compounds can be synthesized:arrow_forwardWhat is the name of the following compound? isopropyl cyclohexylethanoate methylethyl R-cyclohexanecarboxylate methylethyl S-cyclohexanecarboxylate R-methylethyl R-cyclohexylacetate OS-methylethyl S-cyclohexylacetatearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning