
Interpretation:
Oxide of an element that is basic has to be identified from the given options
Concept Introduction:
According to physical and chemical properties, the elements can be further divided into metals, non-metals and metalloids.
In a group, the metallic character of an element increases from top to bottom whereas in a period, it decreases from left to right.
The classification elements in the periodic table as metals, nonmetals, or metalloids can be given as
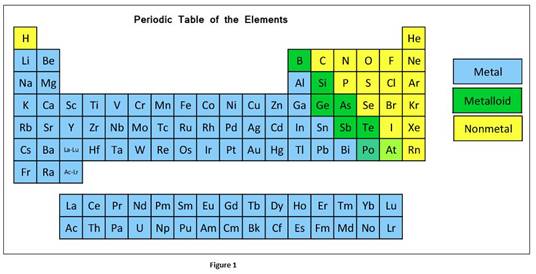
Oxide is a compound which is formed when oxygen reacts with another element. Oxides formed with metals are basic
Oxides formed with metals are most probably basic. Most of oxides formed with nonmetals are acidic.
Amphoteric oxides have the properties of bases and acid. Elements that are in the intermediate position of periodic table form amphoteric oxide.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Loose Leaf for Chemistry
- Phosphate buffers are important in regulating the pH of intracellular fluids. If the concentration ratio of H2PO4/HPO42 in a sample of intracellular fluid is 1.1: 1, what is the pH of this sample of intracellular fluid? H2PO4(aq)HPO42(aq)+H+(aq)Ka=6.2108arrow_forwardThe reaction of calcium hydride, CaH2, with water can be characterized as a Lewis acid-base reaction: CaH2(s)+2H2O(l)Ca(OH)2(aq)+2H2(g) Identify the Lewis acid and the Lewis base among the reactants. The reaction is also an oxidation-reduction reaction. Identify the oxidizing agent, the reducing agent, and the changes in oxidation number that occur in the reaction.arrow_forwardSodium perchlorate, NaClO4, is produced by electrolysis of sodium chlorate, NaClO3. If a current of 2.50 103 A passes through an electrolytic cell, how many kilograms of sodium perchlorate are produced per hour?arrow_forward
- When carbon dioxide dissolves in water it reacts to produce carbonic acid, H2CO3(aq), which can ionize in two steps. H2CO3(aq)HCO3(aq)+H+(aq)Kc1=4.2107HCO3(aq)CO32(aq)+H+(aq)Kc2=4.81011 Calculate the equilibrium constant for the reaction H2CO3(aq)CO32(aq)+2H+(aq)arrow_forwardGiven that: Pb(ClO3)2(aq) + KBr(aq)→ PbBr2(s) + KClO3(aq), which of the following species is classified as a spectator ion?arrow_forwardHypobromous Acid, HOBr, and hypoiodous acid, HOI are both weak acids. Which acid is stronger and why? You may look up the correct Lewis structure for these acids to help you answer the question.arrow_forward
- Would you expect potassium perchlorate, KClO4(aq), in a concentrated acidic solution to act as an oxidizing agent or as a reducing agent?arrow_forwardChemists working with fluorine and its compounds some- times find it helpful to think in terms of acid-base reac- tions in which the fluoride ion (F¯) is donated and ассеpted. (a) Would the acid in this system be the fluoride donor or fluoride acceptor? (b) Identify the acid and base in each of these reactions: CIF;O2 + BF; CIF,O, · BF, -- TiF, + 2 KF – K2[TiF,]arrow_forwardWrite the net ionic equation for the hydrolysis reaction that occurs when sodium carbonate, Na,CO3 is dissolved in water. Predict if the solution will be acidic, basic or neutral.arrow_forward
- The pH of a 0.10 M solution of Ni(NO3)2 is 5.0. Calculate the acid ionization constant of Ni(H 2O) 62+(aq).arrow_forwardUsing Lewis structures, write balanced equations for the following reactions:(a) HCl(g) + PH3(g) ⟶(b) H3 O+ + CH3 − ⟶(c) CaO + SO3 ⟶(d) NH4 + + C2 H5 O− ⟶arrow_forward2- a-treu b-falsearrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





