
Interpretation:
The thermal efficiency of the cycle and the fraction of the steam entering the turbine is extracted for the feed water heater should be determined.
Concept Introduction:
The thermal efficiency of the cycle is calculated as:
η=|Wnet|QH
Here Wnet is the total work produced by the turbine and pump. QH is the difference in enthalpies of the turbine and boiler.
The entropy is constant for idealistic turbine. Hence an isentropic process is developed between the enter and exit pressures of the idealistic turbine. The idealistic condenser has constant enthalpy.
Answer to Problem 8.8P
η=0.311
And
m=0.1868 lbm
Explanation of Solution
Given information:
It is given thatcycle is a regenerative cycle in a steam power plant includes one feedwater heater, turbine, condenser and a boiler. Steam enters the turbine at 650 psia and 900∘F and exhaust at 1 psia to condenser and 50 psia to feedwater heater. Steam for the feedwater heater is at 50 psia and in condensing raises the temperature of the feedwater to within 11∘F . Turbine and pump efficiencies are both given as 0.78 .
Figure to describe the process in simplified form is given as below
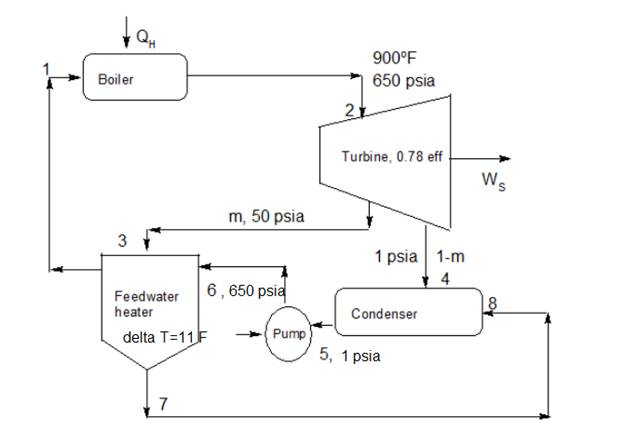
From figure point 1 and point 6 has same operating conditions.
Considering idealistic turbine for which entropy is constant at inlet and outlet
at point 2 and at 650 psia and 900∘F, the properties are written from superheated steam table in fps unit as,
H2=1461.2 Btulbm and S2=1.6671 Btulbm Rankine
Now for idealistic turbine, entropy is constant at inlet and outlet. Hence
S4'
Exhaust coming to condenser is at pressure
The steam coming to condenser is wet and saturated. Hence for saturated steam at
At
At
From linear interpolation, if
Similarly
And
And
We also know that
Therefore,
Hence the actual enthalpy of the steam exhaust from the turbine to the condenser is calculated by the thermodynamics efficiency of the work produced from the turbine which is
Hence
and
Exhaust coming to feedwater heater is at pressure
The steam coming to feedwater heater is superheated. Hence for superheated steam at
From linear interpolation, if
Hence the actual enthalpy of the steam exhaust from the turbine to the feedwater heater is calculated by the thermodynamics efficiency of the work produced from the turbine which is
And entropy at
Now,
The idealistic condenser has constant enthalpy and hence the enthalpy of saturated liquid at inlet point 4 of condenser is same as enthalpy at the outlet of condenser at point 5.
And at
Hence work required for circulation of the steam from the condenser to the feedwater heater is calculated fromusing the pump efficiency is
And the work-done by pump is also calculated as
Now it is given that steam for the feedwater heater is at
Hence at point 7 the steam is saturated liquid from feedwater heater going towards the condenser. Hence for
Hence the temperature at the point 1 according to the given condition and in condensing raises the temperature of the feedwater to within
And at
The volume expansivity for boiler is calculated as
Hence the enthalpy and entropy across the boiler is
And
Let mass of steam exhaust from the turbine going towards feedwater heater is
Basis
Applying energy balance across the feedwater heater. Hence fraction of the steam entering the turbine is extracted for the feedwater heater is
And
The thermal efficiency of the cycle is calculated as:
Here
And
Want to see more full solutions like this?
Chapter 8 Solutions
Introduction to Chemical Engineering Thermodynamics
- 3. Review the figure below and mark it to indicate where relief devices should be placed. Nitrogen Purge Hexane Storage Water Polymer Storage Polymer Steam- HIC HIC (TIC) (TIC Hexane Coolers #FIC) (W) (PIC (PIC) PD Pump Centrifuge #FIC)arrow_forward2. Review the figure below and mark it to indicate where relief devices should be placed. Steam- Nitrogen (Purge Gas) Crude Methanol PD Pump HIC ☑D TIC TC Water TIC Still Steam PD Pump Drum LIC PIC Tars Methanolarrow_forwardCalculate the thickness of the square mica plates (in ft), having an area of 0.01 sq. is falling at a velocity of 5.0 cm through oil with a density of 55 lb/cu ft and with viscosity of 15 centipoise. The specific gravity of the mica is 3.0. Assume that the particle velocity reaches its maximum and so it becomes cement was, and stokes law applies.arrow_forward
- Calculate the thickness of the square mica plates (in ft), having an area of 0.01 sq. is falling at a velocity of 5.0 cm through oil with a density of 55 lb/cu ft and with viscosity of 15 centipoise. The specific gravity of the mica is 3.0. Assume that the particle velocity reaches its maximum and so it becomes cement was, and stokes law applies.arrow_forwardLiquid ammonia (NH₃) is applied as a fertilizer. You are working for a company that applies the ammonia (they charge a lot for every pound they apply to the soil) and you keep track of the pounds of ammonia that has been applied from the tank on the back end of your pickup (four-wheel drive with big tires). After the liquid has been emptied from the tank, there is still gas ammonia left in the tank. It has been sitting in the sun and its temperature is 125°F. Also, the pressure gauge at the top of the tank shows 230 psig (pounds per square inch gauge). The tank has a volume of 120 ft³. How many pounds of ammonia are left in the tank?arrow_forwardn-Hexane is burned with excess air. An analysis of the product gas yields the following dry-basis molar composition: 7.48% CO2, 1.45% CO, 0.556% C6H14 (with the remainder O2 and N2). The stack gas emerges at 760 mm Hg. Calculate the percentage conversion of hexane, the percentage excess air fed to the burner, and the dew point of the stack gas, taking water to be the only condensable species.arrow_forward
- A piston-fitted cylinder with a 5-cm diameter contains 1.65 g of nitrogen. The mass of the piston is 6.50 kg, and a 35.0-kg weight rests on the piston. The gas temperature is 35.0°C, and the pressure outside the cylinder is 3.50 atm. d=5 m₁g N₂ m3kg dcm m2=6.50 m1 1.65 m3 = 35.0 Calculate the pressure in the cylinder. i x 105 Pa eTextbook and Media Save for Later Volume What is the volume of the gas in the cylinder? V = i L m2 kg If the 35.0 kg weight is removed from the piston, how far does the piston move up? i Save for Later m Heat Transferred A How much heat is transferred to (positive) or from (negative) the gas during the expansion? Jarrow_forwardchemical engineering The answer for the specific molar volume of nitrogen gas is 12.089x10^(-5) m^3/mol. How was this answer determined? You need to use the ideal gas law to determine the specific molar volume. Do not determine the third specific enthalpy.arrow_forwardUsing Raoult's law for water and Henry's law for nitrogen, calculate the pressure and gas-phase composition (mole fractions) in a system containing a liquid that is 0.500 mole% N2 and 99.50 mole% water in equilibrium with nitrogen gas and water vapor at 70.0 °C. The Henry's law constant for nitrogen in water is recommended by NIST to be well represented by KH = 0.000625 exp[1300 (1/T - 1/298.15)] mol N2/(kg H2O bar), where T is measured in Kelvin. Physical Property Tables Unit Conversion Check the unit conversions and examine the definition of H. Estimate the Henry's law constant H [atm/(mole fraction N2)] for nitrogen in water at T = 70.0 °C. i ! x 104 atm/(mole fraction N2)arrow_forward
- can you help me answer this, you can find what the data need in perry's chemical engineering handbook 9th editionarrow_forwardchemical engineering. Compressing hydrogen gas from 1 atm to 300 atm at 25 deg Celcius Determine the specific enthalpy.arrow_forwardRotorences] Use the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 73 1.0 72 25 57 8 43 100 29 17 • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. 81 + ་་ CH4 { [ ? Previous Nextarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





