
(a)
Interpretation:
The missing C3H5 and OH substituents needs to be added to the template in order to get one possible chair conformation for each compound.
Concept Introduction:
Chair-like conformation for cyclohexane molecule is a three-dimensional way of representing the molecule. This is a non-planar conformation with all the angles approximately equal to 109.5o. This is stable conformation because it has lower strain energy than the flat hexagonal shape. All the carbon centers are equivalent in the chair conformation. In the flat hexagonal shape, the wedge and dashed bonds represent the group attached directed away and towards the plane of paper respectively. In the chair conformation, these wedge and dashed bonds are axial and equatorial respectively.
(b)
Interpretation:
The most appropriate method from MM2 or MOPAC to determine whether compound 1 or 2 is most stable product in each reaction needs to be determined.
Concept Introduction:
Computational chemistry uses computer simulation to monitor solution of chemical problems. The theoretical chemistry is used in which efficient computer programs are incorporated to determine the structures and properties of molecules.
In the computational chemistry, the MM2 and MOPAC methods are defined as follows:
MM2:
This method is developed mainly for conformational analysis of small organic compounds such as hydrocarbons. This is designed to reproduce the equilibrium covalent geometry of molecules as close as possible.
MOPAC:
This is a computer program used in the computational chemistry for semi-empirical quantum chemistry algorithms. It runs on Mac, Windows and Linux.
(c)
Interpretation:
The number of chair-like conformation for the energy minimization needs to be determined. The alcohol and
Concept Introduction:
Chair-like conformation for cyclohexane molecule is a three-dimensional way of representing the molecule. This is a non-planar conformation with all the angles approximately equal to 109.5o. This is stable conformation because it has lower strain energy than the flat hexagonal shape. All the carbon centers are equivalent in the chair conformation. In the flat hexagonal shape, the wedge and dashed bonds represent the group attached directed away and towards the plane of paper respectively. In the chair conformation, these wedge and dashed bonds are axial and equatorial respectively.
(d)
Interpretation:
The input structure for compound 1 needs to be determined.
Concept Introduction:
Computational chemistry uses computer simulation to monitor solution of chemical problems. The theoretical chemistry is used in which efficient computer programs are incorporated to determine the structures and properties of molecules.
MM2 method is developed mainly for conformational analysis of small organic compounds such as hydrocarbons. This is designed to reproduce the equilibrium covalent geometry of molecules as close as possible.
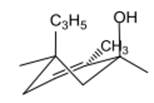
The compound 1 is given as follows:
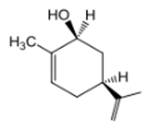
The chair conformation is represented as follows:
Here, both C3H5 and OH groups are at axial positions.
Thus, the input for compound 1 is represented as follows:
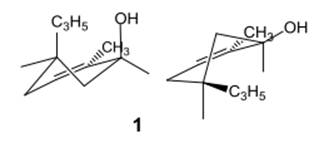
(e)
Interpretation:
The input structure for compound 2 needs to be determined.
Concept Introduction:
Computational chemistry uses computer simulation to monitor solution of chemical problems. The theoretical chemistry is used in which efficient computer programs are incorporated to determine the structures and properties of molecules.
MM2 method is developed mainly for conformational analysis of small organic compounds such as hydrocarbons. This is designed to reproduce the equilibrium covalent geometry of molecules as close as possible.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Laboratory Techniques in Organic Chemistry
- Compound X, C,4H12Br2, is optically inactive. On treatment with strong base, X gives hydrocarbon Y, C14H10: Compound Y absorbs 2 equivalents of hydrogen when reduced over a palladium catalyst to give z (C14H14) and reacts with ozone to give one product, benzoic acid (C,Hg02). Draw the structure of compound Z. • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • Ignore alkene stereochemistry. • If more than one structure fits the description, draw them all. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate structures with + signs from the drop-down menu. ChemDoodlearrow_forwardDraw a structural formula of an alkene or alkenes (if more than one) that undergo acid-catalyzed hydration and without rearrangement give 2-methyl-2-butanol as the MAJOR product. You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. If more than one structure fits the description, draw them all. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu.arrow_forwardDraw the structural formula of the major product of the reaction of (S)-2,2,3-trimethyloxirane with Me,NH. • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • Include H atoms at chiral centers only. • If a group is achiral, do not use wedged or hashed bonds on it.arrow_forward
- Shown below is a carbocation intermediate in an electrophilic addition reaction of HCl with an alkene. For the conformation shown, select each hydrogen whose bond to carbon is aligned for effective hyperconjugation with the vacant p orbital on the positively charged carbon. Each adjacent carbon will have only one C-H bond so aligned. • Gray = C; white = H; red = 0; blue = N; dark green = Cl; brown =Br; light green =F; purple = I; yellow = S; orange = P. • Double click to select atoms. • You can zoom in and out using the mouse scroll wheel (or pinch to zoom on touch screens).arrow_forwardCompound X, C7H15CI, is a chiral product of the radical chlorination of 2-methylhexane. Base-promoted E2 elimination of X gives a single product. What is the structure of X? • Do not use stereobonds in your answer. • In cases where there is more than one possible structure for each molecule, just give one for each. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. . + *** ChemDoodleⓇarrow_forward2. What Alkene would be used to synthesize 3-bromohexane? + HBr CH;CH,CHCH,CH,CH3 br 3. Write down the mechanism for the following reaction and predict which of the two possible products will be formed in the greatest yield ( 2nd and 3d carbocation). Write down the structures of the two possible products and explain your answer. CH;CH = CHCH, CH, CH3 + HBrarrow_forward
- CH3 Br,/FeBr3 C3H;BrO 5 Using resonance structures, justify whether the acetyl group of compound 5 will direct the bromination reaction to the meta or ortho/para positions.arrow_forwardDraw the structures, including stereochemistry, of the major products (A) from the re- action between 1-pentene and mercury(II) acetate in methanol. Assign the configura- tions (R or S) of any stereocenters. Draw the structures, including stereochemistry, of the products (B) obtained from demercuration of A with sodium borohydride (NaBH4); assign the configurations of the stereocenters. Hg(OAc)₂ CH₂OH A NaB H₁ H₂O, EtOH Barrow_forwardHydroboration-oxidation of 2-pentyne gives a mixture of two ketones, each with the molecular formula C5H10O. Propose structural formulas of these two ketones. Explain your proposal.arrow_forward
- (R)-2-butanol reacts with phosphorus tribromide to give A (C4H9Br). Treatment of A with sodium cyanide in DMF gives B (C5H9)N. B is optically active. Draw the structure of B. • Use the wedge/hash bond tools to indicate stereochemistry where it exists. Show stereochemistry in a meso compound. Sn [F ? ChemDoodleⓇarrow_forwardSodium cyanide reacts with 2-bromobutane in dimethylsulfoxide to form a substitution product. Draw the substitution product formed when this enantiomer reacts with NaCN in DMSO. Clearly indicate stereochemistry by drawing a wedged bond, a dashed bond, and two in-plane bonds per each chiral carbon. Then identify the configuration of the starting material and the product.arrow_forward12.Use of KMnO, to effect oxidative cleave of the alkene J, C3H16, yields two fragments, one of which is butanoic acid and the other a ketone, Q. When J reacts with one molar equivalent of H-Cl, the alkyl halide K, C3H17CI, is formed. What are the structures of J, J, and Q? Write all the reactions, and show your reasoning.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





