
(a)
Interpretation:
The given pairs which one is take place more rapid reaction have to be identified.
Concept Introduction:
Nucleophile is a chemical species that gives an electron pair to an electrophile to form a
Charged nucleophile is stronger than neutral nucleophiles.
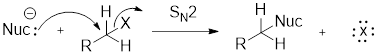
Structure of the substrate plays major role in the reactivity of
(b)
Interpretation:
The given pairs which one is take place more rapid reaction have to be identified.
Concept Introduction:
Nucleophile is a chemical species that gives an electron pair to an electrophile to form a chemical bond in relation to a reaction.
Charged nucleophile is stronger than neutral nucleophiles.
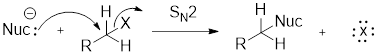
Structure of the substrate plays major role in the reactivity of
(c)
Interpretation:
The given pairs which one is take place more rapid reaction have to be identified.
Concept Introduction:
Nucleophile is a chemical species that gives an electron pair to an electrophile to form a chemical bond in relation to a reaction.
Charged nucleophile is stronger than neutral nucleophiles.
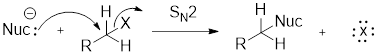
Structure of the substrate plays major role in the reactivity of
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Essential Organic Chemistry (3rd Edition)
- at product will result from the reaction shown? (Just mention the class of compound not the nomenclature) CH3 HOCH,CH,OH CH;CHCH,CH,CH,C-H H+arrow_forwardThe geometry favors the E2 mechanism because it will promote the effectivearrow_forwardPlease review your answers. You say the reaction on the right is slower, then in the next question it is faster?arrow_forward
- Circle the reaction in the pair that will undergo reaction more readily.arrow_forwarda) Consider the reaction of HBr with ethylene and propylene. At roomtemperature the reaction of propylene with HBr is much faster than thereaction with ethylene.Using reaction energy diagrams and your knowledge of carbocationstability explain why this is so. b) Xylene (dimethylbenzene) is a commonly used chemical in the printingindustry and as a cleaning solvent for oily waste. It is also used whenpreparing histological samples to remove waxes from biological samples.Draw the three possible structures for this compound and give the UPACnames for each. Define which structures are ortho, meta, and para.arrow_forwardin this E2 reaction what is the major productarrow_forward
- complete the statement below. At -80 °C, Reaction of HBr with Butadiene, the transition state for the secondary product has a lower Ea because it is a more stable Questarrow_forwardIt is not necessary for a nucleophile to have an unshared electron pair. O True O Falsearrow_forwardWhich of the following is a stronger nucleophile in a polar, protic solvent? OF O HSarrow_forward
- Explain why this reaction is not possible that it cannot yield the provided productarrow_forwardThe rate determining step of a reaction is the slowest step and thus, has the lowest energy barrier first step on a staircase fastest step and thus, has the lowest energy barrier slowest step and thus, has the highest energy barrier fastest step and thus, has the highest energy barrierarrow_forwardDetermine the structures of compounds A and B in the following reaction scheme.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


