
EBK THERMODYNAMICS: AN ENGINEERING APPR
9th Edition
ISBN: 8220106796979
Author: CENGEL
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7.13, Problem 90P
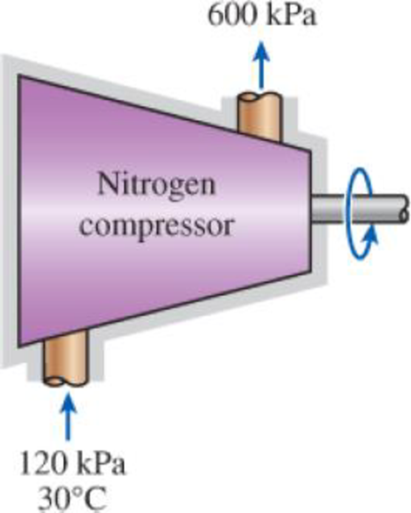
Nitrogen at 120 kPa and 30°C is compressed to 600 kPa in an adiabatic compressor. Calculate the minimum work needed for this process in kJ/kg.
FIGURE P7–90
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
-6-
8 من 8
Mechanical vibration
HW-prob-1
lecture 8 By: Lecturer Mohammed O. attea
The 8-lb body is released from rest a distance xo
to the right of the equilibrium position.
Determine the displacement x as a function of time t,
where t = 0 is the time of release.
c=2.5 lb-sec/ft
wwwww
k-3 lb/in.
8 lb
Prob. -2
Find the value of (c) if the system is critically
damping.
Prob-3
Find Meq and Ceq at point B, Drive eq. of
motion for the system below.
Ш
H
-7~
+
目
T T & T
тт
+
Q For the following plan of building foundation, Determine
immediate settlement at points (A) and (B) knowing that: E,-25MPa,
u=0.3, Depth of foundation (D) =1m, Depth of layer below base level
of foundation (H)=10m.
3m
2m
100kPa
A
2m
150kPa
5m
200kPa
B
W
PE
2
43
R² 80 + 10 + kr³ Ø8=0 +0
R²+J+ kr200
R² + J-) + k r² = 0
kr20
kr20
8+
W₁ =
= 0
R²+1)
R²+J+)
4
lec 8.pdf
Mechanical vibration
lecture 6
By: Lecturer Mohammed C. Attea
HW1 (Energy method)
Find equation of motion and natural frequency for the system shown in fig. by energy
method.
m. Jo
000
HW2// For the system Fig below find
1-F.B.D
2Eq.of motion
8 wn
4-0 (1)
-5-
m
Chapter 7 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
Ch. 7.13 - Does a cycle for which Q 0 violate the Clausius...Ch. 7.13 - Does the cyclic integral of heat have to be zero...Ch. 7.13 - Is a quantity whose cyclic integral is zero...Ch. 7.13 - Prob. 4PCh. 7.13 - Prob. 5PCh. 7.13 - How do the values of the integral 12Q/T compare...Ch. 7.13 - Prob. 7PCh. 7.13 - The entropy of a hot baked potato decreases as it...Ch. 7.13 - When a system is adiabatic, what can be said about...Ch. 7.13 - Prob. 10P
Ch. 7.13 - A pistoncylinder device contains helium gas....Ch. 7.13 - A pistoncylinder device contains nitrogen gas....Ch. 7.13 - A pistoncylinder device contains superheated...Ch. 7.13 - The entropy of steam will (increase, decrease,...Ch. 7.13 - During a heat transfer process, the entropy of a...Ch. 7.13 - Steam is accelerated as it flows through an actual...Ch. 7.13 - Heat is transferred at a rate of 2 kW from a hot...Ch. 7.13 - A completely reversible air conditioner provides...Ch. 7.13 - Heat in the amount of 100 kJ is transferred...Ch. 7.13 - In Prob. 719, assume that the heat is transferred...Ch. 7.13 - During the isothermal heat addition process of a...Ch. 7.13 - Prob. 22PCh. 7.13 - During the isothermal heat rejection process of a...Ch. 7.13 - Air is compressed by a 40-kW compressor from P1 to...Ch. 7.13 - Refrigerant-134a enters the coils of the...Ch. 7.13 - A rigid tank contains an ideal gas at 40C that is...Ch. 7.13 - A rigid vessel is filled with a fluid from a...Ch. 7.13 - A rigid vessel filled with a fluid is allowed to...Ch. 7.13 - Prob. 29PCh. 7.13 - One lbm of R-134a is expanded isentropically in a...Ch. 7.13 - Two lbm of water at 300 psia fill a weighted...Ch. 7.13 - A well-insulated rigid tank contains 3 kg of a...Ch. 7.13 - Using the relation ds = (Q/T)int rev for the...Ch. 7.13 - The radiator of a steam heating system has a...Ch. 7.13 - A rigid tank is divided into two equal parts by a...Ch. 7.13 - Prob. 36PCh. 7.13 - An insulated pistoncylinder device contains 5 L of...Ch. 7.13 - Onekg of R-134a initially at 600 kPa and 25C...Ch. 7.13 - Refrigerant-134a is expanded isentropically from...Ch. 7.13 - Refrigerant-134a at 320 kPa and 40C undergoes an...Ch. 7.13 - A rigid tank contains 5 kg of saturated vapor...Ch. 7.13 - A 0.5-m3 rigid tank contains refrigerant-134a...Ch. 7.13 - Steam enters a steady-flow adiabatic nozzle with a...Ch. 7.13 - Steam enters an adiabatic diffuser at 150 kPa and...Ch. 7.13 - R-134a vapor enters into a turbine at 250 psia and...Ch. 7.13 - Refrigerant-134a enters an adiabatic compressor as...Ch. 7.13 - The compressor in a refrigerator compresses...Ch. 7.13 - An isentropic steam turbine processes 2 kg/s of...Ch. 7.13 - Prob. 52PCh. 7.13 - Twokg of saturated water vapor at 600 kPa are...Ch. 7.13 - A pistoncylinder device contains 5 kg of steam at...Ch. 7.13 - Prob. 55PCh. 7.13 - In Prob. 755, the water is stirred at the same...Ch. 7.13 - Prob. 57PCh. 7.13 - Prob. 58PCh. 7.13 - Determine the total heat transfer for the...Ch. 7.13 - Calculate the heat transfer, in kJ/kg. for the...Ch. 7.13 - Prob. 61PCh. 7.13 - An adiabatic pump is to be used to compress...Ch. 7.13 - Prob. 63PCh. 7.13 - Prob. 64PCh. 7.13 - A 30-kg aluminum block initially at 140C is...Ch. 7.13 - A 50-kg copper block initially at 140C is dropped...Ch. 7.13 - A 30-kg iron block and a 40-kg copper block, both...Ch. 7.13 - Prob. 69PCh. 7.13 - Prob. 70PCh. 7.13 - Can the entropy of an ideal gas change during an...Ch. 7.13 - An ideal gas undergoes a process between two...Ch. 7.13 - Prob. 73PCh. 7.13 - Air is expanded from 200 psia and 500F to 100 psia...Ch. 7.13 - Prob. 75PCh. 7.13 - Air is expanded isentropically from 100 psia and...Ch. 7.13 - Which of the two gaseshelium or nitrogenhas the...Ch. 7.13 - Which of the two gasesneon or airhas the lower...Ch. 7.13 - A 1.5-m3 insulated rigid tank contains 2.7 kg of...Ch. 7.13 - An insulated pistoncylinder device initially...Ch. 7.13 - A pistoncylinder device contains 0.75 kg of...Ch. 7.13 - A mass of 25 lbm of helium undergoes a process...Ch. 7.13 - One kg of air at 200 kPa and 127C is contained in...Ch. 7.13 - An insulated rigid tank is divided into two equal...Ch. 7.13 - Air at 27C and 100 kPa is contained in a...Ch. 7.13 - Air at 3.5 MPa and 500C is expanded in an...Ch. 7.13 - Air is compressed in a pistoncylinder device from...Ch. 7.13 - Helium gas is compressed from 90 kPa and 30C to...Ch. 7.13 - Nitrogen at 120 kPa and 30C is compressed to 600...Ch. 7.13 - Five kg of air at 427C and 600 kPa are contained...Ch. 7.13 - Prob. 92PCh. 7.13 - Prob. 93PCh. 7.13 - Prob. 94PCh. 7.13 - The well-insulated container shown in Fig. P 795E...Ch. 7.13 - An insulated rigid tank contains 4 kg of argon gas...Ch. 7.13 - Prob. 97PCh. 7.13 - Prob. 98PCh. 7.13 - Prob. 99PCh. 7.13 - It is well known that the power consumed by a...Ch. 7.13 - Calculate the work produced, in kJ/kg, for the...Ch. 7.13 - Prob. 102PCh. 7.13 - Prob. 103PCh. 7.13 - Saturated water vapor at 150C is compressed in a...Ch. 7.13 - Liquid water at 120 kPa enters a 7-kW pump where...Ch. 7.13 - Water enters the pump of a steam power plant as...Ch. 7.13 - Consider a steam power plant that operates between...Ch. 7.13 - Saturated refrigerant-134a vapor at 15 psia is...Ch. 7.13 - Helium gas is compressed from 16 psia and 85F to...Ch. 7.13 - Nitrogen gas is compressed from 80 kPa and 27C to...Ch. 7.13 - Describe the ideal process for an (a) adiabatic...Ch. 7.13 - Is the isentropic process a suitable model for...Ch. 7.13 - On a T-s diagram, does the actual exit state...Ch. 7.13 - Argon gas enters an adiabatic turbine at 800C and...Ch. 7.13 - Steam at 100 psia and 650F is expanded...Ch. 7.13 - Combustion gases enter an adiabatic gas turbine at...Ch. 7.13 - Steam at 4 MPa and 350C is expanded in an...Ch. 7.13 - Prob. 120PCh. 7.13 - Prob. 121PCh. 7.13 - Refrigerant-134a enters an adiabatic compressor as...Ch. 7.13 - The adiabatic compressor of a refrigeration system...Ch. 7.13 - Prob. 125PCh. 7.13 - Argon gas enters an adiabatic compressor at 14...Ch. 7.13 - Prob. 127PCh. 7.13 - Air enters an adiabatic nozzle at 45 psia and 940F...Ch. 7.13 - An adiabatic diffuser at the inlet of a jet engine...Ch. 7.13 - Hot combustion gases enter the nozzle of a...Ch. 7.13 - The exhaust nozzle of a jet engine expands air at...Ch. 7.13 - Prob. 133PCh. 7.13 - Refrigerant-134a is expanded adiabatically from...Ch. 7.13 - A frictionless pistoncylinder device contains...Ch. 7.13 - Prob. 136PCh. 7.13 - Steam enters an adiabatic turbine steadily at 7...Ch. 7.13 - Prob. 138PCh. 7.13 - Oxygen enters an insulated 12-cm-diameter pipe...Ch. 7.13 - Water at 20 psia and 50F enters a mixing chamber...Ch. 7.13 - Prob. 141PCh. 7.13 - Prob. 142PCh. 7.13 - In a dairy plant, milk at 4C is pasteurized...Ch. 7.13 - Steam is to be condensed in the condenser of a...Ch. 7.13 - An ordinary egg can be approximated as a...Ch. 7.13 - Prob. 146PCh. 7.13 - In a production facility, 1.2-in-thick, 2-ft 2-ft...Ch. 7.13 - Prob. 148PCh. 7.13 - Prob. 149PCh. 7.13 - Prob. 150PCh. 7.13 - Prob. 151PCh. 7.13 - Prob. 152PCh. 7.13 - Prob. 153PCh. 7.13 - Liquid water at 200 kPa and 15C is heated in a...Ch. 7.13 - Prob. 155PCh. 7.13 - Prob. 157PCh. 7.13 - Prob. 158PCh. 7.13 - Prob. 159PCh. 7.13 - Prob. 160PCh. 7.13 - The compressed-air requirements of a plant are met...Ch. 7.13 - Prob. 162PCh. 7.13 - The space heating of a facility is accomplished by...Ch. 7.13 - Prob. 164PCh. 7.13 - Prob. 165PCh. 7.13 - Prob. 166PCh. 7.13 - Prob. 167RPCh. 7.13 - A refrigerator with a coefficient of performance...Ch. 7.13 - What is the minimum internal energy that steam can...Ch. 7.13 - Prob. 170RPCh. 7.13 - What is the maximum volume that 3 kg of oxygen at...Ch. 7.13 - A 100-lbm block of a solid material whose specific...Ch. 7.13 - Prob. 173RPCh. 7.13 - A pistoncylinder device initially contains 15 ft3...Ch. 7.13 - A pistoncylinder device contains steam that...Ch. 7.13 - Prob. 176RPCh. 7.13 - Prob. 177RPCh. 7.13 - Prob. 178RPCh. 7.13 - A 0.8-m3 rigid tank contains carbon dioxide (CO2)...Ch. 7.13 - Air enters the evaporator section of a window air...Ch. 7.13 - Prob. 181RPCh. 7.13 - Prob. 182RPCh. 7.13 - Prob. 183RPCh. 7.13 - Prob. 184RPCh. 7.13 - Helium gas is throttled steadily from 400 kPa and...Ch. 7.13 - Determine the work input and entropy generation...Ch. 7.13 - Prob. 187RPCh. 7.13 - Reconsider Prob. 7187. Determine the change in the...Ch. 7.13 - Prob. 189RPCh. 7.13 - Air enters a two-stage compressor at 100 kPa and...Ch. 7.13 - Three kg of helium gas at 100 kPa and 27C are...Ch. 7.13 - Steam at 6 MPa and 500C enters a two-stage...Ch. 7.13 - Prob. 193RPCh. 7.13 - Prob. 194RPCh. 7.13 - Refrigerant-134a enters a compressor as a...Ch. 7.13 - Prob. 196RPCh. 7.13 - Prob. 197RPCh. 7.13 - Prob. 198RPCh. 7.13 - Prob. 199RPCh. 7.13 - Prob. 200RPCh. 7.13 - Prob. 201RPCh. 7.13 - Prob. 202RPCh. 7.13 - Prob. 203RPCh. 7.13 - Prob. 204RPCh. 7.13 - Prob. 205RPCh. 7.13 - Prob. 206RPCh. 7.13 - Prob. 207RPCh. 7.13 - Prob. 208RPCh. 7.13 - (a) Water flows through a shower head steadily at...Ch. 7.13 - Prob. 211RPCh. 7.13 - Prob. 212RPCh. 7.13 - Prob. 213RPCh. 7.13 - Consider the turbocharger of an internal...Ch. 7.13 - Prob. 215RPCh. 7.13 - Prob. 216RPCh. 7.13 - A 5-ft3 rigid tank initially contains...Ch. 7.13 - Prob. 218RPCh. 7.13 - Show that the difference between the reversible...Ch. 7.13 - Demonstrate the validity of the Clausius...Ch. 7.13 - Consider two bodies of identical mass m and...Ch. 7.13 - Consider a three-stage isentropic compressor with...Ch. 7.13 - Prob. 223RPCh. 7.13 - Prob. 224RPCh. 7.13 - Prob. 225RPCh. 7.13 - The polytropic or small stage efficiency of a...Ch. 7.13 - Steam is condensed at a constant temperature of...Ch. 7.13 - Steam is compressed from 6 MPa and 300C to 10 MPa...Ch. 7.13 - An apple with a mass of 0.12 kg and average...Ch. 7.13 - A pistoncylinder device contains 5 kg of saturated...Ch. 7.13 - Argon gas expands in an adiabatic turbine from 3...Ch. 7.13 - A unit mass of a substance undergoes an...Ch. 7.13 - A unit mass of an ideal gas at temperature T...Ch. 7.13 - Heat is lost through a plane wall steadily at a...Ch. 7.13 - Air is compressed steadily and adiabatically from...Ch. 7.13 - Argon gas expands in an adiabatic turbine steadily...Ch. 7.13 - Water enters a pump steadily at 100 kPa at a rate...Ch. 7.13 - Air is to be compressed steadily and...Ch. 7.13 - Helium gas enters an adiabatic nozzle steadily at...Ch. 7.13 - Combustion gases with a specific heat ratio of 1.3...Ch. 7.13 - Steam enters an adiabatic turbine steadily at 400C...Ch. 7.13 - Liquid water enters an adiabatic piping system at...Ch. 7.13 - Liquid water is to be compressed by a pump whose...Ch. 7.13 - Steam enters an adiabatic turbine at 8 MPa and...Ch. 7.13 - Helium gas is compressed steadily from 90 kPa and...Ch. 7.13 - Helium gas is compressed from 1 atm and 25C to a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- The hose supplying the cylinder operating the bucket of a large excavator has fluid at 1000 psi flowing at 5 gpm. What is theavailable power in the line?arrow_forwardQ For the following plan of building foundation, Determine immediate settlement at points (A) and (B) knowing that: E,-25MPa, u=0.3, Depth of foundation (D) =1m, Depth of layer below base level of foundation (H)=10m. 3m 2m 100kPa A 2m 150kPa 5m 200kPa Barrow_forwardGiven the following data for crack rocker mechanism. If θ2 = 4π/3 and ω2 = 1 rad/s, Determine all possible values of ω4 and ω3 analytically. The lengths of links are a = 2, b = 8, c = 7 and d = 9 in cm.arrow_forward
- Q6] (20 Marks) Select the most suitable choice for the following statements: modo digi -1A 10 af5 1 -The copper-based alloy which is responded to age hardening is a) copper-nickel b) aluminum bronze c) copper - beryllium d) brass besincaluy 2- Highly elastic polymers may experience elongations to greater than.... b) 500% bromsia-P c) 1000%. d) 1200% 15m or -2 a)100% 3- The cooling rate of quenching the steel in saltwater will be ......the cooling rate of quenching ir c) faster than sold) none of them a) slower than 4- Adding of a) Cr b) the same as ...... Will lead to stabilize the b) Mo 10 austenite in steel. c) Nimble avolls 1d) Sized loloin nl 5- The adjacent linear chains of crosslinked polymers are joined one to another at various positic DIR... by.........bonds c) covalent noisqo gd) ionic lg 120M 6- For the ceramic with coordination number 6 the cation to anion radius ratio will be a) Van der Waals a) 0.155-0.225 a) linear b) hydrogen (b) 0.225-0.414 c) 0.414 0.732 ..polymers.…arrow_forwardExamine Notes: Attempt Six Questions Only. rever necessa , Q1] (20 Marks) Answer with true (T) or false (F), corrects the wrong phrases, and gives sho reasons for correct and corrected statements: 1- High chromium irons are basically grey cast irons alloyed with 12 to 30 % Cr. yous board-19qgo orT-1 2- The drawbacks of Al- Li alloys are their high young modulus and high density.&M 0) (0 3- Vulcanized rubbers are classified under thermoplastic polymers. 4- Diamond is a stable carbon polymorph at room temperature and atmospheric pressure. ( 5- The metallic ions of ceramic are called anions, and they are positively charged. yldgiH-S 69001(6arrow_forwardH.W 5.4 Calculate the load that will make point A move to the left by 6mm, E-228GPa. The diameters of the rods are as shown in fig. below. 2P- PA 50mm B 200mm 2P 0.9m 1.3marrow_forward
- d₁ = = Two solid cylindrical road AB and BC are welded together at B and loaded as shown. Knowing that 30mm (for AB) and d₂ 50mm (for BC), find the average normal stress in each road and the total deformation of road AB and BC. E=220GPa H.W 5.3 60kN A For the previous example calculate the value of force P so that the point A will not move, and what is the total length of road AB at that force? P◄ A 125kN 125kN 0.9m 125kN 125kN 0.9m B B 1.3m 1.3marrow_forwardClass: B Calculate the load that will make point A move to the left by 6mm, E-228GPa The cross sections of the rods are as shown in fig. below. 183 P- Solution 1.418mm 200mm 80mm 3P- 18.3 A 080mm B 200mm 3P- 0.9m إعدادات العرض 1.3m 4.061mmarrow_forwardH.W6 Determine the largest weight W that can be supported by two wires shown in Fig. P109. The stress in either wire is not to exceed 30 ksi. The cross- sectional areas of wires AB and AC are 0.4 in2 and 0.5 in2, respectively. 50° 30° Warrow_forward
- Find equation of motion and natural frequency for the system shown in fig. by energy method. H.W2// For the system Fig below find 1-F.B.D 2-Eq.of motion 8wn 4-0 (5) m. Jo marrow_forward2. Read the following Vernier caliper measurements. (The scales have been enlarged for easier reading.) The Vernier caliper is calibrated in metric units. (a) 0 1 2 3 4 5 سلسلسله (b) 1 2 3 4 5 6 سلسل (c) 1 23456 (d) 1 2 3 4 5 6 سلسلسarrow_forwardExplain why on the interval 0<x<1000 mm and 1000<x<2000mm, Mt is equal to positive 160 Nm, but at x= 0mm and x=1000mm Mt is equal to -160 Nm (negative value!). What is the reason for the sign change of Mt?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Thermodynamic Availability, What is?; Author: MechanicaLEi;https://www.youtube.com/watch?v=-04oxjgS99w;License: Standard Youtube License