
EBK THERMODYNAMICS: AN ENGINEERING APPR
9th Edition
ISBN: 8220106796979
Author: CENGEL
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7.13, Problem 32P
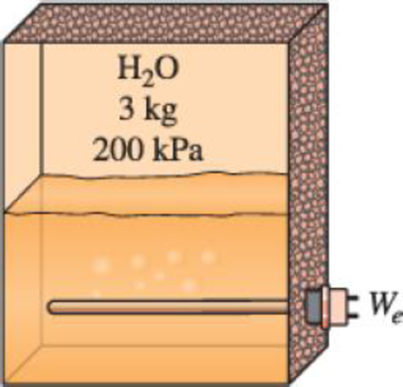
A well-insulated rigid tank contains 3 kg of a saturated liquid–vapor mixture of water at 200 kPa. Initially, three-quarters of the mass is in the liquid phase. An electric resistance heater placed in the tank is now turned on and kept on until all the liquid in the tank is vaporized. Determine the entropy change of the steam during this process.
FIGURE P7–32
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A well-insulated rigid tank contains 3 kg of a saturated liquid–vapor mixture of water at 200 kPa. Initially, threequarters of the mass is in the liquid phase. An electric resistance heater placed in the tank is now turned on and kept on until all the liquid in the tank is vaporized. Determine the entropy change of the steam during this process
A 0.5-m³ rigid tank contains refrigerant-134a initially at 250 kPa and 45 percent quality. Heat is
transferred now to the refrigerant from a source at 35°C until the pressure rises to 450 kPa.
Determine:
a. The entropy change of the refrigerant.
b. The entropy change of the heat source.
C.
The total entropy change for this process.
Using EES software, investigate the effect of the source temperature and final pressure on the total
entropy change for the process. Let the source temperature vary from 30°C to 210°C, and the final
pressure vary from 300 to 500kPa. Plot the total entropy change for the process as a function of the
source temperature for final pressures of 300 kPa, 400 kPa, and 500 kPa, and discuss the results.
Onekg of R-134a initially at 600 kPa and 25°C undergoes a process during which the entropy is kept constant until the pressure drops to 100 kPa. Determine the final temperature of the R-134a and the final specific internal energy
Chapter 7 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
Ch. 7.13 - Does a cycle for which Q 0 violate the Clausius...Ch. 7.13 - Does the cyclic integral of heat have to be zero...Ch. 7.13 - Is a quantity whose cyclic integral is zero...Ch. 7.13 - Prob. 4PCh. 7.13 - Prob. 5PCh. 7.13 - How do the values of the integral 12Q/T compare...Ch. 7.13 - Prob. 7PCh. 7.13 - The entropy of a hot baked potato decreases as it...Ch. 7.13 - When a system is adiabatic, what can be said about...Ch. 7.13 - Prob. 10P
Ch. 7.13 - A pistoncylinder device contains helium gas....Ch. 7.13 - A pistoncylinder device contains nitrogen gas....Ch. 7.13 - A pistoncylinder device contains superheated...Ch. 7.13 - The entropy of steam will (increase, decrease,...Ch. 7.13 - During a heat transfer process, the entropy of a...Ch. 7.13 - Steam is accelerated as it flows through an actual...Ch. 7.13 - Heat is transferred at a rate of 2 kW from a hot...Ch. 7.13 - A completely reversible air conditioner provides...Ch. 7.13 - Heat in the amount of 100 kJ is transferred...Ch. 7.13 - In Prob. 719, assume that the heat is transferred...Ch. 7.13 - During the isothermal heat addition process of a...Ch. 7.13 - Prob. 22PCh. 7.13 - During the isothermal heat rejection process of a...Ch. 7.13 - Air is compressed by a 40-kW compressor from P1 to...Ch. 7.13 - Refrigerant-134a enters the coils of the...Ch. 7.13 - A rigid tank contains an ideal gas at 40C that is...Ch. 7.13 - A rigid vessel is filled with a fluid from a...Ch. 7.13 - A rigid vessel filled with a fluid is allowed to...Ch. 7.13 - Prob. 29PCh. 7.13 - One lbm of R-134a is expanded isentropically in a...Ch. 7.13 - Two lbm of water at 300 psia fill a weighted...Ch. 7.13 - A well-insulated rigid tank contains 3 kg of a...Ch. 7.13 - Using the relation ds = (Q/T)int rev for the...Ch. 7.13 - The radiator of a steam heating system has a...Ch. 7.13 - A rigid tank is divided into two equal parts by a...Ch. 7.13 - Prob. 36PCh. 7.13 - An insulated pistoncylinder device contains 5 L of...Ch. 7.13 - Onekg of R-134a initially at 600 kPa and 25C...Ch. 7.13 - Refrigerant-134a is expanded isentropically from...Ch. 7.13 - Refrigerant-134a at 320 kPa and 40C undergoes an...Ch. 7.13 - A rigid tank contains 5 kg of saturated vapor...Ch. 7.13 - A 0.5-m3 rigid tank contains refrigerant-134a...Ch. 7.13 - Steam enters a steady-flow adiabatic nozzle with a...Ch. 7.13 - Steam enters an adiabatic diffuser at 150 kPa and...Ch. 7.13 - R-134a vapor enters into a turbine at 250 psia and...Ch. 7.13 - Refrigerant-134a enters an adiabatic compressor as...Ch. 7.13 - The compressor in a refrigerator compresses...Ch. 7.13 - An isentropic steam turbine processes 2 kg/s of...Ch. 7.13 - Prob. 52PCh. 7.13 - Twokg of saturated water vapor at 600 kPa are...Ch. 7.13 - A pistoncylinder device contains 5 kg of steam at...Ch. 7.13 - Prob. 55PCh. 7.13 - In Prob. 755, the water is stirred at the same...Ch. 7.13 - Prob. 57PCh. 7.13 - Prob. 58PCh. 7.13 - Determine the total heat transfer for the...Ch. 7.13 - Calculate the heat transfer, in kJ/kg. for the...Ch. 7.13 - Prob. 61PCh. 7.13 - An adiabatic pump is to be used to compress...Ch. 7.13 - Prob. 63PCh. 7.13 - Prob. 64PCh. 7.13 - A 30-kg aluminum block initially at 140C is...Ch. 7.13 - A 50-kg copper block initially at 140C is dropped...Ch. 7.13 - A 30-kg iron block and a 40-kg copper block, both...Ch. 7.13 - Prob. 69PCh. 7.13 - Prob. 70PCh. 7.13 - Can the entropy of an ideal gas change during an...Ch. 7.13 - An ideal gas undergoes a process between two...Ch. 7.13 - Prob. 73PCh. 7.13 - Air is expanded from 200 psia and 500F to 100 psia...Ch. 7.13 - Prob. 75PCh. 7.13 - Air is expanded isentropically from 100 psia and...Ch. 7.13 - Which of the two gaseshelium or nitrogenhas the...Ch. 7.13 - Which of the two gasesneon or airhas the lower...Ch. 7.13 - A 1.5-m3 insulated rigid tank contains 2.7 kg of...Ch. 7.13 - An insulated pistoncylinder device initially...Ch. 7.13 - A pistoncylinder device contains 0.75 kg of...Ch. 7.13 - A mass of 25 lbm of helium undergoes a process...Ch. 7.13 - One kg of air at 200 kPa and 127C is contained in...Ch. 7.13 - An insulated rigid tank is divided into two equal...Ch. 7.13 - Air at 27C and 100 kPa is contained in a...Ch. 7.13 - Air at 3.5 MPa and 500C is expanded in an...Ch. 7.13 - Air is compressed in a pistoncylinder device from...Ch. 7.13 - Helium gas is compressed from 90 kPa and 30C to...Ch. 7.13 - Nitrogen at 120 kPa and 30C is compressed to 600...Ch. 7.13 - Five kg of air at 427C and 600 kPa are contained...Ch. 7.13 - Prob. 92PCh. 7.13 - Prob. 93PCh. 7.13 - Prob. 94PCh. 7.13 - The well-insulated container shown in Fig. P 795E...Ch. 7.13 - An insulated rigid tank contains 4 kg of argon gas...Ch. 7.13 - Prob. 97PCh. 7.13 - Prob. 98PCh. 7.13 - Prob. 99PCh. 7.13 - It is well known that the power consumed by a...Ch. 7.13 - Calculate the work produced, in kJ/kg, for the...Ch. 7.13 - Prob. 102PCh. 7.13 - Prob. 103PCh. 7.13 - Saturated water vapor at 150C is compressed in a...Ch. 7.13 - Liquid water at 120 kPa enters a 7-kW pump where...Ch. 7.13 - Water enters the pump of a steam power plant as...Ch. 7.13 - Consider a steam power plant that operates between...Ch. 7.13 - Saturated refrigerant-134a vapor at 15 psia is...Ch. 7.13 - Helium gas is compressed from 16 psia and 85F to...Ch. 7.13 - Nitrogen gas is compressed from 80 kPa and 27C to...Ch. 7.13 - Describe the ideal process for an (a) adiabatic...Ch. 7.13 - Is the isentropic process a suitable model for...Ch. 7.13 - On a T-s diagram, does the actual exit state...Ch. 7.13 - Argon gas enters an adiabatic turbine at 800C and...Ch. 7.13 - Steam at 100 psia and 650F is expanded...Ch. 7.13 - Combustion gases enter an adiabatic gas turbine at...Ch. 7.13 - Steam at 4 MPa and 350C is expanded in an...Ch. 7.13 - Prob. 120PCh. 7.13 - Prob. 121PCh. 7.13 - Refrigerant-134a enters an adiabatic compressor as...Ch. 7.13 - The adiabatic compressor of a refrigeration system...Ch. 7.13 - Prob. 125PCh. 7.13 - Argon gas enters an adiabatic compressor at 14...Ch. 7.13 - Prob. 127PCh. 7.13 - Air enters an adiabatic nozzle at 45 psia and 940F...Ch. 7.13 - An adiabatic diffuser at the inlet of a jet engine...Ch. 7.13 - Hot combustion gases enter the nozzle of a...Ch. 7.13 - The exhaust nozzle of a jet engine expands air at...Ch. 7.13 - Prob. 133PCh. 7.13 - Refrigerant-134a is expanded adiabatically from...Ch. 7.13 - A frictionless pistoncylinder device contains...Ch. 7.13 - Prob. 136PCh. 7.13 - Steam enters an adiabatic turbine steadily at 7...Ch. 7.13 - Prob. 138PCh. 7.13 - Oxygen enters an insulated 12-cm-diameter pipe...Ch. 7.13 - Water at 20 psia and 50F enters a mixing chamber...Ch. 7.13 - Prob. 141PCh. 7.13 - Prob. 142PCh. 7.13 - In a dairy plant, milk at 4C is pasteurized...Ch. 7.13 - Steam is to be condensed in the condenser of a...Ch. 7.13 - An ordinary egg can be approximated as a...Ch. 7.13 - Prob. 146PCh. 7.13 - In a production facility, 1.2-in-thick, 2-ft 2-ft...Ch. 7.13 - Prob. 148PCh. 7.13 - Prob. 149PCh. 7.13 - Prob. 150PCh. 7.13 - Prob. 151PCh. 7.13 - Prob. 152PCh. 7.13 - Prob. 153PCh. 7.13 - Liquid water at 200 kPa and 15C is heated in a...Ch. 7.13 - Prob. 155PCh. 7.13 - Prob. 157PCh. 7.13 - Prob. 158PCh. 7.13 - Prob. 159PCh. 7.13 - Prob. 160PCh. 7.13 - The compressed-air requirements of a plant are met...Ch. 7.13 - Prob. 162PCh. 7.13 - The space heating of a facility is accomplished by...Ch. 7.13 - Prob. 164PCh. 7.13 - Prob. 165PCh. 7.13 - Prob. 166PCh. 7.13 - Prob. 167RPCh. 7.13 - A refrigerator with a coefficient of performance...Ch. 7.13 - What is the minimum internal energy that steam can...Ch. 7.13 - Prob. 170RPCh. 7.13 - What is the maximum volume that 3 kg of oxygen at...Ch. 7.13 - A 100-lbm block of a solid material whose specific...Ch. 7.13 - Prob. 173RPCh. 7.13 - A pistoncylinder device initially contains 15 ft3...Ch. 7.13 - A pistoncylinder device contains steam that...Ch. 7.13 - Prob. 176RPCh. 7.13 - Prob. 177RPCh. 7.13 - Prob. 178RPCh. 7.13 - A 0.8-m3 rigid tank contains carbon dioxide (CO2)...Ch. 7.13 - Air enters the evaporator section of a window air...Ch. 7.13 - Prob. 181RPCh. 7.13 - Prob. 182RPCh. 7.13 - Prob. 183RPCh. 7.13 - Prob. 184RPCh. 7.13 - Helium gas is throttled steadily from 400 kPa and...Ch. 7.13 - Determine the work input and entropy generation...Ch. 7.13 - Prob. 187RPCh. 7.13 - Reconsider Prob. 7187. Determine the change in the...Ch. 7.13 - Prob. 189RPCh. 7.13 - Air enters a two-stage compressor at 100 kPa and...Ch. 7.13 - Three kg of helium gas at 100 kPa and 27C are...Ch. 7.13 - Steam at 6 MPa and 500C enters a two-stage...Ch. 7.13 - Prob. 193RPCh. 7.13 - Prob. 194RPCh. 7.13 - Refrigerant-134a enters a compressor as a...Ch. 7.13 - Prob. 196RPCh. 7.13 - Prob. 197RPCh. 7.13 - Prob. 198RPCh. 7.13 - Prob. 199RPCh. 7.13 - Prob. 200RPCh. 7.13 - Prob. 201RPCh. 7.13 - Prob. 202RPCh. 7.13 - Prob. 203RPCh. 7.13 - Prob. 204RPCh. 7.13 - Prob. 205RPCh. 7.13 - Prob. 206RPCh. 7.13 - Prob. 207RPCh. 7.13 - Prob. 208RPCh. 7.13 - (a) Water flows through a shower head steadily at...Ch. 7.13 - Prob. 211RPCh. 7.13 - Prob. 212RPCh. 7.13 - Prob. 213RPCh. 7.13 - Consider the turbocharger of an internal...Ch. 7.13 - Prob. 215RPCh. 7.13 - Prob. 216RPCh. 7.13 - A 5-ft3 rigid tank initially contains...Ch. 7.13 - Prob. 218RPCh. 7.13 - Show that the difference between the reversible...Ch. 7.13 - Demonstrate the validity of the Clausius...Ch. 7.13 - Consider two bodies of identical mass m and...Ch. 7.13 - Consider a three-stage isentropic compressor with...Ch. 7.13 - Prob. 223RPCh. 7.13 - Prob. 224RPCh. 7.13 - Prob. 225RPCh. 7.13 - The polytropic or small stage efficiency of a...Ch. 7.13 - Steam is condensed at a constant temperature of...Ch. 7.13 - Steam is compressed from 6 MPa and 300C to 10 MPa...Ch. 7.13 - An apple with a mass of 0.12 kg and average...Ch. 7.13 - A pistoncylinder device contains 5 kg of saturated...Ch. 7.13 - Argon gas expands in an adiabatic turbine from 3...Ch. 7.13 - A unit mass of a substance undergoes an...Ch. 7.13 - A unit mass of an ideal gas at temperature T...Ch. 7.13 - Heat is lost through a plane wall steadily at a...Ch. 7.13 - Air is compressed steadily and adiabatically from...Ch. 7.13 - Argon gas expands in an adiabatic turbine steadily...Ch. 7.13 - Water enters a pump steadily at 100 kPa at a rate...Ch. 7.13 - Air is to be compressed steadily and...Ch. 7.13 - Helium gas enters an adiabatic nozzle steadily at...Ch. 7.13 - Combustion gases with a specific heat ratio of 1.3...Ch. 7.13 - Steam enters an adiabatic turbine steadily at 400C...Ch. 7.13 - Liquid water enters an adiabatic piping system at...Ch. 7.13 - Liquid water is to be compressed by a pump whose...Ch. 7.13 - Steam enters an adiabatic turbine at 8 MPa and...Ch. 7.13 - Helium gas is compressed steadily from 90 kPa and...Ch. 7.13 - Helium gas is compressed from 1 atm and 25C to a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- An insulated free-moving piston-cylinder device contains 0.5L of saturated liquid water at a constant pressure of 100 kPa. An electric resistance heater inside the cylinder is now turned on, and 1178.36 kJ of electrical work is transferred to the steam. Determine (a) the entropy change of the water during this process; (b) the entropy generation during this process? 1178.36 klarrow_forwardA 0.6-m rigid tank contains refrigerant-134a initially at 200 kPa and 40 percent quality. Heat is transferred now to the refrigerant from a source at 35°C until the pressure rises to 400 kPa. Determine the entropy change of the refrigerant. Use the tables for R-134a. (You must provide an answer before moving on to the next part.) kJ/K The entropy change of the refrigerant isarrow_forwardBaal eyels A 0.8-m insulated rigid tank contains 2 kg of carbon dioxide at 90 kPa. Now paddle- wheel work is done on the system until the pressure in the tank rises to 150 kPa. Determine the minimum paddle-wheel work of this process. Take To =300 K (R = 0.189RJ %3D 0.68 J kgK kgK FO Uring constant specific heats at roomarrow_forward
- An insulated piston-cylinder device contains 0.5L of saturated liquid water at a constant pressure of 100 kPa. An electric resistance heater inside the cylinder is now turned on, and 1178.36 kJ of electrical work is transferred to the steam. Determine (a) the entropy change of the water during this process; (b) the entropy generation during this process? 1178.36 kJ M H₂O 100 kPa Sat. liquidarrow_forwardA 0.5-m3 rigid tank contains refrigerant-134a initially at 200 kPa and 40 percent quality. Heat is transferred now to the refrigerant from a source at 35°C until the pressure rises to 400 kPa. Determine the entropy change of the refrigerant.arrow_forwardWater vapor at 100 kPa and 150°C is compressed isothermally until half the vapor has condensed. How much work must be performed on the steam in this compression process in kW if the mass flow rate is 4.968 kg/s?arrow_forward
- A rigid tank is divided into two equal parts by a partition. One part of the tank contains 1.5 kg of compressed liquid water at 400 kPa and 60°C while the other part is evacuated. The partition is now removed, and the water expands to fill the entire tank. Determine the entropy change of water during this process, if the final pressure in the tank is 40 kPa. Use steam tables. compressed liquid 400 kPa 60°C Vacuum The entropy change of water during this process is KJ/K.arrow_forwardA piston-cylinder device contains 0.13 kg of air initially at 2 MPa and 350°C. The air is first expanded isothermally to 500 kPa, then compressed polytropically with a polytropic exponent of 1.2 to the initial pressure, and finally compressed at the constant pressure to the initial state. Determine the boundary work for each process and the net work of the cycle. The properties of air are R = 0.287 kJ/kg-K and k = 1.4. The boundary work for the isothermal expansion process is The boundary work for the polytropic compression process is The boundary work for the constant pressure compression process is The net work for the cycle is kJ. kJ. KJ. kJ.arrow_forwardA 0.5-m3 rigid tank contains refrigerant-134a initially at 200 kPa and 40 percent quality. Heat is transferred now to the refrigerant from a source at 358C until the pressure rises to 400 kPa. Determine (a) the entropy change of the refrigerant, (b) the entropy change of the heat source, and (c) the total entropy change for this process.arrow_forward
- A 0.5-m3 rigid tank contains refrigerant-134a initially at 200 kPa and 40 percent quality. Heat is transferred now to the refrigerant from a source at 35°C until the pressure rises to 400 kPa. Determine the total entropy change for this process.arrow_forwardIn a closed container of constant volume, there is initially 39 kg refrigerant at 50 ° C and 100 kPa pressure. Then the refrigerant is cooled until the pressure is 90kPa. Calculate the entropy change in the refrigerant during this process.arrow_forwardthermodynamicsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Thermodynamic Availability, What is?; Author: MechanicaLEi;https://www.youtube.com/watch?v=-04oxjgS99w;License: Standard Youtube License