
Concept explainers
Interpretation:
The wavelength, initial quantum number and the final quantum number in the visible spectrum of hydrogen have to be labeled.
Concept Introduction:
The wave nature of any light can be described by its frequency, wavelength, and amplitude. The wavelength
The relation between frequency
Here,
The relation between the energy
Here,
The energy of Bohr orbits of hydrogen is dependent on the principal quantum number
Answer to Problem 7.40QE
The labeled visible spectrum of hydrogen is as follows:
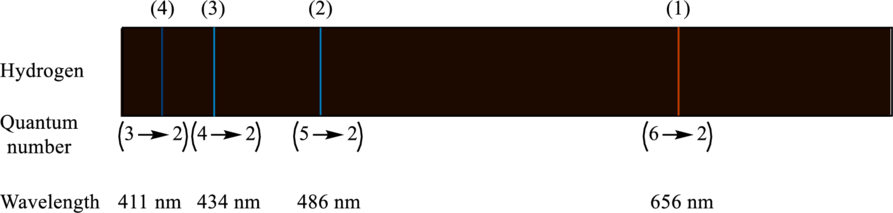
Explanation of Solution
The visible spectrum of hydrogen gives four lines that represent four transitions of the hydrogen as follows:
(1) Transition 1 is from
(2) Transition 1 is from
(3) Transition 1 is from
(4) Transition 1 is from
The expression to calculate the energy difference between initial Bohr orbit
Substitute
Hence, the expression to calculate energy difference between initial Bohr orbit
Here,
For transition (1):
Substitute 3 for
Substitute
Rearrange equation (6) to calculate the value of
Substitute
For transition (2):
Substitute 4 for
Substitute
For transition (3):
Substitute 5 for
Substitute
For transition (4):
Substitute 6 for
Substitute
Want to see more full solutions like this?
Chapter 7 Solutions
Chemistry: Principles and Practice
- A fellow chemistry student says that low-frequency radiation is short-wavelength radiation. You disagree. Explain why the other student is wrong.arrow_forwardSome scientists study Rydberg atoms, atoms whose electron has a large value of the n quantum number. Some Rydberg hydrogen atoms may have consequences in interstellar chemistry. Predict the radius of a Rydberg hydrogen atom that has n=100.arrow_forwardWhat is the wavelength of a baseball having mass 100.0g traveling at a speed of 160km/hr? What is the wavelength of an electron traveling at the same speed?arrow_forward
- Suppose an atom in an excited state can return to the ground state in two steps. It first falls to an intermediate state, emitting radiation of wavelength 1 , and then to the ground state, emitting radiation of wavelength 2 . The same atom can also return to the ground state in one step, with the emission of radiation of wavelength . How are 1,2, and related? How are the frequencies of the three radiations related?arrow_forwardExplain the hydrogen emission spectrum. Why is it significant that the calm emitted is not while? How does the emission spectrum support the idea of quantized energy levels?arrow_forwarda For a pendulum having classical frequency of 1.00s1, what is the energy difference in J between quantized energy levels? b Calculate the wavelength of light that must be absorbed in order for the pendulum to go from one level to another. c Can you determine in what region of the electromagnetic spectrum such a wavelength belongs? d Comment on your results for parts a and b based on your knowledge of the state of science in early twentieth century. Why wasnt the quantum mechanical behavior of nature noticed?arrow_forward
- Photons are emitted in the Lyman series as hydrogen atoms undergo transitions from various excited states to the ground state. If ground-state He+ are present in the same gas (near stars, for example), can they absorb these photons? Explain.arrow_forwardLight of very long wavelength strikes a photosensitive metallic surface and no electrons are ejected. Explain why increasing the intensity of this light on the metal still will not cause the photoelectric effect.arrow_forwardAssume that an electron can absorb more than one photon in the photoelectric effect. a How many photons of wavelength 776.5nm does an electron in Fe need to absorb to escape the iron surface ((Fe)=4.67eV)? b What is the resulting velocity of the emitted electron?arrow_forward
- In 1885, Johann Balmer, a mathematician, derived the following relation for the wavelength of lines in the visible spectrum of hydrogen =364.5 n2( n2 4) where in nanometers and n is an integer that can be 3, 4, 5, . . . Show that this relation follows from the Bohr equation and the equation using the Rydberg constant. Note that in the Balmer series, the electron is returning to the n=2 level.arrow_forwardThe energy needed to ionize an atom of element X when it is in its most stable state is 500kJmol1 . However, if an atom of X is in its lowest excited state, only 120kJmol1 is needed to ionize it. What is the wavelength of the radiation emitted when an atom of X undergoes a transition from the lowest excited state to the ground state?arrow_forwardA piano tuner uses a tuning fork that emits sound with a frequency of 440s1 . Calculate the wavelength of the sound from this tuning fork and the time the sound takes to travel 10.0 m across a large room. Take the speed of sound in air to be 343ms1 .arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning  Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





