
Concept explainers
(a)
Interpretation:
Whether the given substrate undergoes E2 elimination step with base
Concept introduction:
Answer to Problem 7.29P
The given substrate cannot undergo E2 elimination step with base
Explanation of Solution
The given substrate is
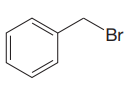
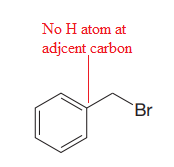
Thus, the given substrate cannot undergo
No H atom on adjacent carbon atom.
(b)
Interpretation:
Whether the given substrate undergoes E2 elimination step with base
Concept introduction:
Answer to Problem 7.29P
The given substrate undergoes E2 elimination step.
The E2 elimination step for the given substrate is drawn as:
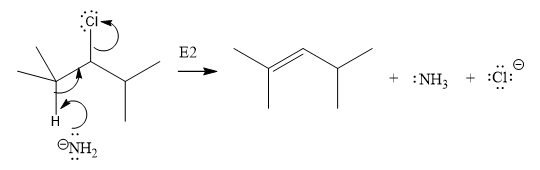
Explanation of Solution
The given substrate is:
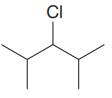
In the above given substrate, Cl acts as a leaving group with one H atom on each side. Any one of the hydrogen atom is taken away forming a C=C bond. The first curved arrow is drawn from the lone pair of nitrogen atom of a base

The product of
(c)
Interpretation:
Whether the given substrate undergo E2 elimination step with base
Concept introduction:
Answer to Problem 7.29P
The given substrate undergoes E2 elimination step.
The E2 elimination step for the given substrate is drawn as:
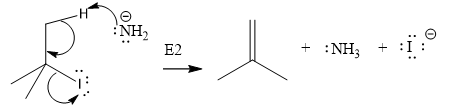
Explanation of Solution
The given substrate is:
In the above given substrate,
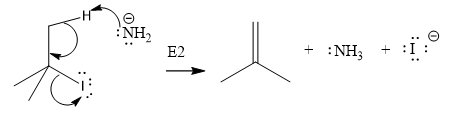
The product of
(d)
Interpretation:
Whether the given substrate undergoes E2 elimination step with base
Concept introduction:
Answer to Problem 7.29P
The given substrate cannot undergoes E2 elimination step with base
Explanation of Solution
The given substrate is
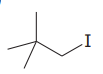
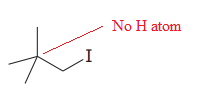
Thus, the given substrate cannot undergo
There is no H atom on the adjacent carbon atom.
(e)
Interpretation:
Whether the given substrate undergo E2 elimination step with base
Concept introduction:
The
Answer to Problem 7.29P
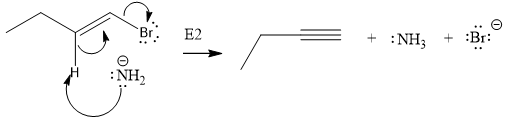
The given substrate undergoes E2 elimination step.
The E2 elimination step for the given substrate is drawn as:
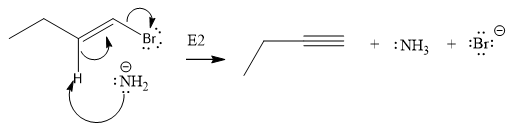
Explanation of Solution
The given substrate is
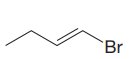
In the above given substrate,
The product of
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry: Principles And Mechanisms
- So, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forwardDraw a mental model for calcium chloride mixed with sodium phosphatearrow_forward
- here is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forwardDrawing of 3-fluro-2methylphenolarrow_forward
- Which compound(s) will be fully deprotonated (>99%) by reaction with one molar equivalent of sodium hydroxide? I, II, III I, || I, III I only II, III SH | H3C-C=C-H || III NH2arrow_forwardWill NBS (and heat or light) work for this reaction, or do we have to use Br2?arrow_forwardHAND DRAWarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
