
Organic Chemistry, Loose-leaf Version
8th Edition
ISBN: 9781305865549
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 6.3, Problem 6.3P
Interpretation Introduction
Interpretation:
The carbocations should be arranged in increasing stability order.
Concept Introduction:
Carbocation: The carbon ion that bears a positive charge on it is termed as carbocation.
Carbocation stability order:
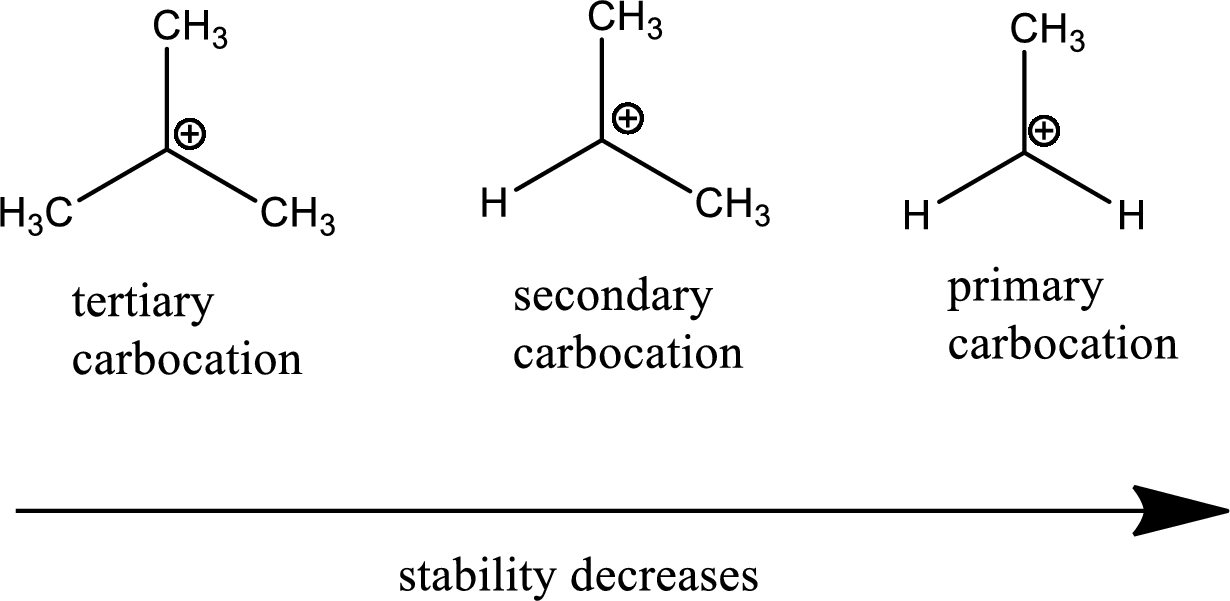
The tertiary carbocation is more stable than secondary and primary since tertiary carbocation contains 3 methyl groups (electron releasing) in which the positive charge is distributed. The more positive charge gets distributed, more stable the compound. Therefore, the tertiary is more stable than secondary and secondary is more stable than primary.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Rank the following alkenes in order of increasing stability
1. (a) Describe aromaticity, Kekule structure and resonance structure for benzene.
(b) Why is benzene more stable than aliphatic alkenes?
Draw all alkenes formed from alkyl halide, and predict the major product.
Chapter 6 Solutions
Organic Chemistry, Loose-leaf Version
Ch. 6.2 - Using the BDE values from Appendix 3, calculate...Ch. 6.3 - Name and draw a structural formula for the product...Ch. 6.3 - Prob. 6.3PCh. 6.3 - Propose a mechanism for the addition of HI to...Ch. 6.3 - Prob. 6.5PCh. 6.3 - Propose a mechanism for the acid-catalyzed...Ch. 6.3 - The acid-catalyzed hydration of...Ch. 6.3 - Complete these reactions. (a) (b)Ch. 6.3 - Draw the structure of the chlorohydrin formed by...Ch. 6.4 - Draw structural formulas for the alkene that gives...
Ch. 6.5 - Prob. 6.11PCh. 6.5 - Prob. 6.12PCh. 6.5 - What alkene with the molecular formula C6H12, when...Ch. 6 - Prob. 6.15PCh. 6 - Prob. 6.16PCh. 6 - Predict the organic product(s) of the reaction of...Ch. 6 - Prob. 6.18PCh. 6 - Prob. 6.20PCh. 6 - Draw a structural formula for an alkene with the...Ch. 6 - Account for the fact that addition of HCl to...Ch. 6 - Account for the fact that treating propenoic acid...Ch. 6 - Draw a structural formula for the alkene with the...Ch. 6 - Draw the alternative chair conformations for the...Ch. 6 - Draw a structural formula for the cycloalkene with...Ch. 6 - Reaction of this bicycloalkene with bromine in...Ch. 6 - Terpin, prepared commercially by the...Ch. 6 - Propose a mechanism for this reaction and account...Ch. 6 - Treating 2-methylpropene with methanol in the...Ch. 6 - When 2-pentene is treated with Cl2 in methanol,...Ch. 6 - Treating cyclohexene with HBr in the presence of...Ch. 6 - Propose a mechanism for this reaction. 1-Pentane...Ch. 6 - Treating 4-penten-1-ol with bromine in water forms...Ch. 6 - Prob. 6.35PCh. 6 - Prob. 6.36PCh. 6 - Reaction of -pinene with borane followed by...Ch. 6 - Write structural formulas for the major organic...Ch. 6 - Draw the structural formula of the alkene that...Ch. 6 - Consider the following reaction. (a) Draw a...Ch. 6 - Prob. 6.42PCh. 6 - Prob. 6.43PCh. 6 - Show how to convert ethylene to these compounds....Ch. 6 - Show how to convert cyclopentene into these...Ch. 6 - Prob. 6.46PCh. 6 - Describe the stereochemistry of the bromohydrin...Ch. 6 - Prob. 6.49PCh. 6 - Treating 1,3-butadiene with 1 mole of HBr gives a...Ch. 6 - In this chapter, we studied the mechanism of the...Ch. 6 - As we have seen in this chapter, carbon-carbon...Ch. 6 - Prob. 6.53PCh. 6 - Prob. 6.54P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H H Write the structures of the alkenes that would yield the following carbonyl compounds PRACTICE PROBLEM 8.22 when treated with ozone and then with dimethyl sulfide. (a) and (c) EO and `H oalio (2 mol is produced from 1 mol of alkene) (b) H.arrow_forward4.1 9 (a) Define geometric isomerism (b) How does geometric isomerism occur in alkenes? (c) Draw the cis- and trans- geometric isomers (if they exist) of: (i) BrCH=CHBr (ii) CH,CH,CH=CHCH,CH, (iii) Br- CH₂ - CH₂ - Brarrow_forwardG.306.arrow_forward
- For each molecular formula, draw all the isomeric alkynes, and give their IUPAC names.Circle the acetylenic hydrogen of each terminal alkyne.(a) C5H8 (three isomers)arrow_forward1 Provide the IUPAC name of the following compounds, with clear indication of stereochemistry for stereocenters and alkene. (a) (b) Br (c) Me. (d) Mearrow_forward1. Name the following molecules according to the IUPAC nomenclature system. Indicate stereochemistry where appropriate. (a) CH2OCH,CH3 Br Cl CH3 oSH (b) Clarrow_forward
- Telfairine, a naturally occurring insecticide, and halomon, an antitumor agent, are two polyhalogenated compounds isolated from red algae. (a) Classify each halide bonded to an sp3 hybridized carbon as 1°, 2°, or 3°. (b) Label each halide as vinyl, allylic, or neither.arrow_forwardArrange the alkenes in each set in order of increasing rate of reaction with HI and explain the basis for your ranking. Draw the structural formula of the major product formed in each case. (a) and (b) H3CH₂CHC=CH₂ and (H3C) 2C=CHCH3arrow_forward4.12arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY