
Concept explainers
(a)
Interpretation:
The given
Concept Introduction:
Carbocation: it is carbon ion that bears a positive charge on it.
Leaving group: it is a fragment that leaves from a substrate with a pair of electrons via heterolytic bond cleavage.
Carbocation stability order:
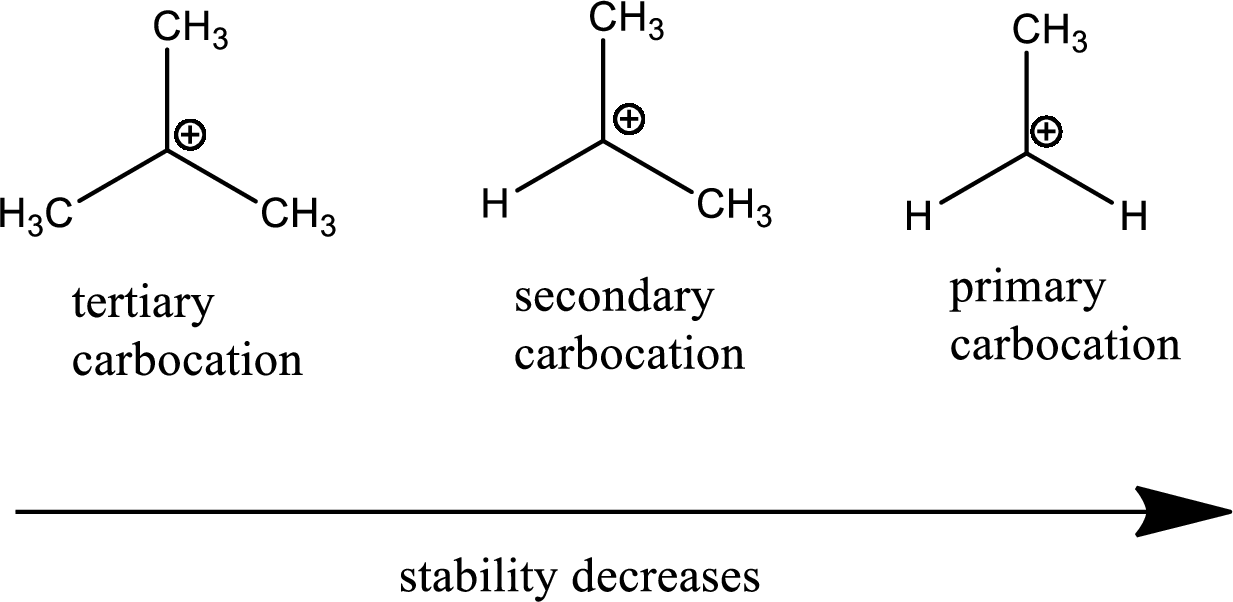
Resonance stabilization: Due to the delocalization of electrons within the molecule the overall energy becomes lower and makes that molecule more stable.
Nucleophile: Nucleophiles are species that donate an electron pair and are also termed as electron-hating species.
Electrophile: Electrophiles are species that accept an electron pair and are also termed as electron-loving species.
(b)
Interpretation:
The given alkenes should be arranged in increasing rate when they react with
Concept Introduction:
Carbocation: it is carbon ion that bears a positive charge on it.
Leaving group: it is a fragment that leaves from a substrate with a pair of electrons via heterolytic bond cleavage.
Carbocation stability order:
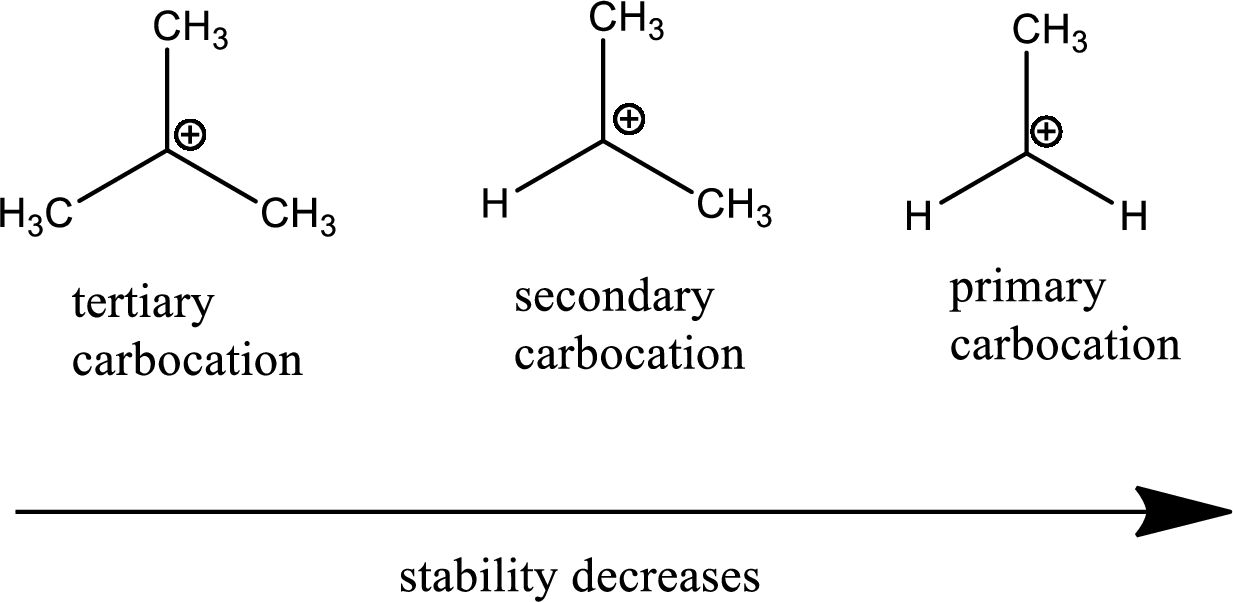
Resonance stabilization: Due to the delocalization of electrons within the molecule the overall energy becomes lower and makes that molecule more stable.
Nucleophile: Nucleophiles are species that donate an electron pair and are also termed as electron-hating species.
Electrophile: Electrophiles are species that accept an electron pair and are also termed as electron-loving species.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry, Loose-leaf Version
- Ethers can be prepared by reaction of an alkoxide or phenoxide ion with a primary alkyl halide. Draw the structure of the expected organic product of the reaction of iodomethane with the following alkoxide ion: CH3 H3C O Na You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • Do not include counter-ions, e.g., Na", I, in your answer. орy вste ChemDoodlearrow_forwardNAME and DRAW the STRUCTURE of ALL the alkenes which could undergo catalytic hydrogenation at 900°C to form methylcyclopentane. Circle the alkene with the HIGHEST stability and X the alkene with the HIGHEST heat of hydrogenation. Give reasons for your choice.arrow_forwardDraw the structural formula of the enol formed in each alkyne hydration reaction; then draw the structural formula of the carbonyl compound with which each enol is in equilibriumarrow_forward
- 1) 3- Methyl-2-butanol will react with H2SO4 to give two isomeric alkenes. Write suitable reaction mechanism to show how these isomeric products are achieved and indicate the major product. Write the IUPAC name for each product.arrow_forwardA synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forwardDescribe how 1-ethylcyclohexanol can be prepared from cyclohexane. You can use any inorganic reagents, any solvents, and any organic reagents as long as they contain no more than two carbons.arrow_forward
- When cyclohexene is reacted with hydrobromic acid in acetic acid, the major product is bromocyclohexane. There is a small amount of cyclohexyl acetate formed. What is the mechanism that forms both compounds? What is the purification procedure that isolates both compounds?arrow_forwardDraw all possible products formed when 2-methylbut-2-ene undergoes addition with HCl. Label them as being either the major or the minor product.arrow_forwardUsing your reaction roadmap as a guide, show how to convert cyclohexane into hexanedial. Show all reagents and all molecules synthesized along the way.arrow_forward
- Draw the structure of the major product of benzene + CH3CH2CH2Cl in AlCl3arrow_forwardWyerone, a compound found in broad beans, has antifungal properties. A Wyerone Identify a trans and a cis alkene in wyerone. Explain the distinction. Draw the product that would be formed by addition of Br2 to the double bond labelled A. Draw the product formed by addition of H2 to wyerone using Lindlar's catalyst. Draw the product formed by addition of excess H2 to wyerone using Pd metal as catalyst.arrow_forwardThe major product of the following reaction exists as two stereoisomers. Draw both isomers: show all hydrogen atoms in the structures. Use wedge and dash bonds to indicate the stereochemistry (you don’t need to draw wedge and dash bonds for C-H bonds of CH3 and CH2 groups). Assign stereo configuration of the asymmetric carbon atoms and write the relationship between two isomers.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

